Review: New oral anticoagulants reduced stroke and systemic
advertisement

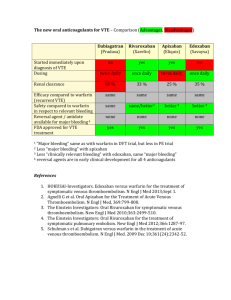
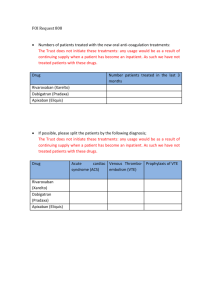
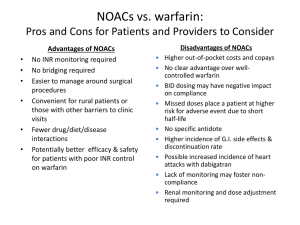
Journal Club – September 2012 Coagulation – Brief Review Brief Review: Warfarin • Blocks vitamin Kdependent glutamate carboxylation of precursor factors II, VII, IX, X • Vit K = cofactor • Warfarin blocks the reduction of Vit K • Oral administration Review: Heparin • • • • Indirect thrombin inhibitor: IV only Called UFH (unfractionated heparin) Complexes with AT (heparin co-factor I) AT by itself inactivates SLOWLY! – Thrombin – Factor Xa – XIIa, XIa, IXa (lesser extent) • AT + Heparin: conformational change in AT = 1000-4000 fold acceleration in inactivation • At high concentrations: Also binds to platelets and heparin co-factor II—which inhibits thrombin Limitations of Warfarin and Heparin: • Both have narrow therapeutic windows • Highly variable dose responses: requires laboratory monitoring (PT, APTT) – Heparin can bind to other plasma proteins making bioavailability variable – Warfarin has numerous food, drug interactions • Limited ability to stop a clot from propagating: – Heparin does not inactivate thrombin bound to fibrin or Xa bound to platelets very well LMWHs • Molecular wt: Heparin: 15,000 vs LMWH: 40005000 • LMWHs inactivate Xa but have less effect on thrombin (some molecules not long enough) – ratio of anti-Xa to anti-thrombin activity of 3:1 – Do not prolong PTT unless dose high • Advantages over heparin: – Easier to administer: sq, BID dosing – Dosage and anticoagulant effect easier to predict; dose based on body weight – Lab monitoring not necessary in all patients – Less chance of inducing immunemediated thrombocytopenia New Anticoagulants • Dabigatran • Fondaparinux Idraparinux • Rivaroxaban Apixaban • LY517717 • YM150 • DU-176b • Betrixaban • TAK 442 New Anticoagulants ORAL PARENTERAL TF/VIIa TTP889 TFPI (tifacogin) X Rivaroxaban Apixaban LY517717 YM150 DU-176b Betrixaban TAK 442 IX VIIIa Va Xa APC (drotrecogin alfa) sTM (ART-123) IXa AT II Dabigatran DX-9065a IIa Fibrinogen Fondaparinux Idraparinux Fibrin Direct Thrombin inhibition Tissue factor XIIa XIa VIIa IXa Xa II × Factor IIa (thrombin) Dabigatran Direct Factor Xa inhibition XIIa XIa IXa × Xa Factor II (prothrombin) Fibrinogen Fibrin clot Tissue factor VIIa Rivaroxaban Apixaban YM150 DU-176b LY517717 Betrixaban TAK 442 Apixaban Oral, direct, selective factor Xa inhibitor Produces concentration-dependent anticoagulation No formation of reactive intermediates No organ toxicity or LFT abnormalities in chronic toxicology studies Low likelihood of drug interactions or QTc prolongation Good oral bioavailability No food effect Balanced elimination (~25% renal) Half-life ~12 hrs He et al., ASH, 2006, Lassen, et al ASH, 2006 O N NH2 N O O N N O Rivaroxaban: oral direct Factor Xa inhibitor Predictable pharmacology High bioavailability Low risk of drug–drug interactions Fixed dose No requirement for monitoring Perzborn et al. 2005; Kubitza et al. 2005; 2006; 2007; Roehrig et al, 2005 O O N O N O Cl S H N O Rivaroxaban® – rivaroxaban Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453-60. In patients with atrial fibrillation (AF), are new oral anticoagulants effective and safe for preventing stroke and systemic embolism compared with warfarin? Scope of Review Included studies compared a new, non–vitamin K antagonist oral anticoagulant with warfarin in patients with AF, had >1 year follow-up, and were published in peer-reviewed journals. Primary efficacy outcome was a composite of stroke (including hemorrhagic stroke) and systemic embolism. Secondary efficacy outcomes were ischemic and unidentified stroke, hemorrhagic stroke, all-cause mortality, vascular mortality, and myocardial infarction. Primary safety outcome was major bleeding; secondary safety outcomes were gastrointestinal bleeding and intracranial bleeding. Review Method MEDLINE, EMBASE/Excerpta Medica, Cochrane Library, Science Citation Index Expanded, and ProQuest’s Dissertations and Theses database (all to Jul 2011) clinical trials databases; reviews; and reference lists were searched for randomized controlled trials (RCTs). 3 noninferiority RCTs met the selection criteria: (n= 44 563, mean age 70 to 73 y, 60% to 65% men, median follow-up 657 to 730 d) ARISTOTLE (n= 18 201) assessed apixaban RE-LY (n= 18 113) assessed dabigatran ROCKET-AF (n= 14 264) assessed rivaroxaban. Results Results Results - Summary Conclusions The new oral anticoagulants reduced the risk for a composite end point of stroke and systemic embolism compared to warfarin. New oral anticoagulants were also found to be associated with a lower risk for key secondary efficacy outcomes, including ischemic and unidentified stroke, hem-orrhagic stroke, all-cause mortality, and vascular mortality, compared to warfarin The review was inconclusive with respect to major bleeding and gastrointestinal bleeding but found a substantial decrease in the risk for intracranial bleeding. Overall, the results support the use of the new oral anticoagulants as alternatives to warfarin for longterm anticoagulation therapy in patients with AF. Comments (1) The highest benefit for prevention of stroke and emboli in patients at moderate risk was with dabigatran (150 mg) Rivaroxaban seemed best suited for patients at highest risk. The safest bleeding profile was achieved with apixaban or dabigatran (110 mg - a dose that is unavailable in the US) The only drug that showed a significant decrease in major bleeding was apixaban. Comments (2) Dabigatran is contraindicated in patients with renal dysfunction. Apixaban is safe for patients with moderate renal disease. Patients with creatinine clearance <30 mL/min should use warfarin. Once-daily rivaroxaban is more convenient than twice-daily dabigatran or apixaban. Patients on warfarin with good control of international normalized ratio (>70% of time in thera-peutic range) or those with valvular AF should continue to use warfarin. Comments (3) There are no specific treatments to reverse the new anticoagulants during bleeding events, although dabigatran may be dialyzable and prothrombin complex concentrates to reverse apixaban and rivaroxaban are being evaluated. Individualized drug selection is important and can be achieved by using risk stratification schemes for thrombosis (CHA2DS 2 -VASc) and bleeding (HASBLED). Thank you !!