ZEISS AXIOSKOP
advertisement

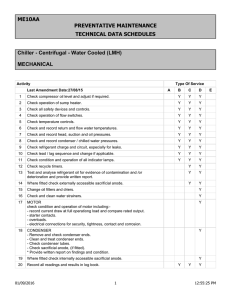
ZEISS AXIOSKOP Microscope’s User Manual Managed by For information about this instrument, please contact Dr. Alloysius Budi Utama @ ext. 8232 or e-mail budiutama@rice.edu Use of the Axioskop (Zeiss) The Axioskop is equipped for viewing samples with transmitted light (from a halogen lamp source) and has optics for: Bright field, Phase Contrast and Differential Interference Contrast (DIC) microscopy. Samples can also be viewed for fluorescence microscopy using the incidentlight system (front a mercury lamp source). 1c 1) For transmitted light microscopy work, press the green on/off light switch (1a) at the back-right side of the microscope while light intensity / level knob is located next to switch (1b). The halogen lamp is in the vented box at the bottom rear of the scope (1c). 1a 1b 2) For fluorescence microscopy work, press green on/off light switch (2a) on the front of the arc lamp power supply box behind the scope (right side- 2b). The mercury lamp is in the vented housing on the rear of the scope and is above the halogen lamp box (2c). *Note: the halogen lamp does not need to be on when only viewing fluorescence 2c 2b 2b 2a Transmitted light microscopy (Brightfield/DIC) Begin with glass slide/specimen securely in place on stage, light at medium brightness/temperature (~6). Ocular's (eyepieces) diopter rings set at "white dot-to-0-position". In most cases start with 10 x objective lens in position. Obtain a "basic" focus using the coarse (large inner black knob), and then if desired, the fine (smaller outer knob) coaxial focusing control knobs (on the back left and right side of the microscope base). *Note: one revolution of the course knob = ~2 mm of stage travel toward any Z-direction Kohler Alignment To achieve maximum even illumination of the sample, the condenser needs to be properly focused and adjusted (Kohler Alignment) 1) Put the specimen in focus in the light path, then pull out the Analyzer [thin black plastic slider with dark filter that is below and to right of the ocular and the large black filter slider]. Turn/rotate counter-clockwise the lower Polarizer using its Pull out black lever (at the Analyzer bottom of the condenser and above the field diaphragm) so they are not in the light path. *Note: for Brightfield/DIC adjustment, the black horizontal metal filter slider should be in the "no-filter/ open position" (last position on left or 5th slot to left; pulled to the extreme right). **Note: After adjustment, for Brightfield use, you can leave analyzer & bottom polarizer in OUT position. For DIC use, analyzer and polarizer can be returned (slide IN) to the light path. 2) Transmitted light can be controlled by adjusting the black circular field diaphragm lens (3a) at the base of the microscope by turning its outer ring clockwise (opens) or counterclockwise (closes). To adjust the condenser and focus the light, put the sample on the stage look into the ocular and focus it. 3a 3b 3c 3d 3b Then close the field diaphragm, center the "small" light spot by turning the left and right silver color centering screws (3b - Two silver screws at 7 & 5 o'clock on the front of the condenser). Raise /lower the condenser apparatus by turning the small black knob (3c - on the left back side of the condenser below the stage) until the circumference of the light image is "sharp and well defined as a hexagon shape." Sharp edge Before After * Note: During this process, the particular condenser ring optics can be selected to match the type of imaging desired: turn the condenser (turret) control ring (3d) to: HDIC for brightfield work; DIC for differential interference contrast work. For Phase Contrast work, use no “1” for 10X, no “2” for 20X and 40X and no “3” for 63X objective. DIC Brightfield Phase 1 Phase 3 **Note: it might not be necessary to adjust/focus the condenser for different objective lenses (magnifications). For example, if condenser is focused for 20x, that could be "acceptable" for 10x and 40x (air objectives); focus for 63x could be "acceptable" for 100x (oil objectives), but NOT for the 10, 20, and 40x air. Minor re-centering with the condenser centering screws can be performed: the light is best centered when the spot is opened up with the field diaphragm ring to about 80- 90% full field illumination, and the light circumference is "concentric" with the edge of the field boundary. ***Note: the silver ring on the condenser opens (clockwise) and closes (counter-clockwise) a diaphragm for more or less light, respectively — lesser light might give best image (totally counter-clockwise), however, adjust as necessary for the conditions. The best DIC images are determined empirically, and important factors are: positions of the prism (adjusted by turning the little silver screw above each objective lens – red arrows in left image) and the condenser aperture diaphragm. Turn the prism screw for the desired image, and turn the diaphragm ring – 3a [more open diaphragm gives more contrast and depth of focus]. On the right side of the microscope base, there are 4 buttons for the filter magazine in the illuminating beam path, and from front-to-back are: neutral density filters 0.015, 0.06, 0.25, and a conversion filter which converts artificial halogen light (color temperature of 3200 K) into natural outdoor light (color temperature of 5500 K). When push buttons are IN or OUT, the corresponding filters are IN or OUT as well. Fluorescence microscopy After turning ON the mercury arch lamp, record in the log hook the number on the time counter and actual time of day. * Note: once the arc lamp is ON, it must stay on for at least 30 minutes before being turned off— when the lamp is turned OFF, it must remain off (to cool) for at least 30 minutes before being turned ON again. Pull out Analyzer filter. ** Note: Pull out the analyzer (thin black plastic slider with dark filter which is below and to the right of the ocular and the large black metal filter slider) from the light path because it not only blocks light, but continued exposure to UV light will damage the When the arc lamp is turned on, and the slide/specimen is placed in position on the stage, illumination of the sample from the objective lens will be readily apparent. The slider, black and marked L on left side and R on right side, can be used to block / interrupt the illumination beam path (move to far right position) when not viewing the sample to prevent photobleaching. The green filter/middle position slightly filters the light, but the open position (far right) is probably best when examining the sample. *Note: in fluorescence microscopy, the less exposure of the sample / fluorophore to excitation light, the less photobleaching and subsequent loss of fluorescence and intensity of signal. The fluorescence reflector or tiller slider [large horizontal black metal box between ocular and objective lenses] has 4 positions for fluorescence that contain various excitation filter / dichroic mirror / emission filter sets, from right to left: // ~450-490 um ex. (green em.) // ~510-560 nm ex. (red em.) // ~450-490 nm ex. (green/yellow em) // ~300-390 nm ex. (blue em.) Decide which filter set is appropriate/best for exciting and detecting the fluorophore(s) being used, and move the filter slider to that position. [See fluorescence reflector slider sheet for filter set details.] *Note: It should not be necessary to change/adjust the illumination beam, however, the following controls affect the diameter and centering of the light. Levers for adjusting the luminous field diaphragm (upper, left and right side) — move by pushing or pulling levers to the right to reduce and to the left to increase the illuminated area [“normally," the area of light should just fill the field of view, as for the halogen lamp beam with light microscopy]. Use the two silver centering screws (lower, left and right side) to position the luminous field diaphragm - to move and align the light beam, alternate twisting both screws. Zeiss Axioskop Fluorescence Reflector Slider The first (right-most) position: (for FITC/ AF488/ GFP - type fluorophores) Excitation filter: 450 - 490 nm Dichroic mirror: 495 nm Emission filter: 500 - 550 nm Second position: (for Rhodamine/ AF594/ Texas red - type fluorophores) Excitation filter: 510- 560 nm Dichroic mirror: 580 nm Emission filter: 590 nm LP Third position: (for YFP - type fluorophores) Excitation filter: 450 - 490 nm Dichroic mirror: 510 nm Emission filter: 520 nm LP Fourth position: (for DAPI / Hoeschst - type fluorophores) Excitation filter: 300(365)390 nm Dichroic mirror: 395 nm Emission filter: 420 nm LP Fifth position: (for DIC / Brightfield) OPEN (no filters) Objectives for Zeiss Axioskop Plan - NEOFLUAR 10X, NA 03 Ph 1 (air; n=1) Phase contrast: condenser position 1 Brightfield: condenser position HDIC DIC - contrast: condenser position DIC Plan - NEOFLUAR 20X, NA 0.5 Ph 2 (air; n=1) Phase contrast: condenser position 2 Brightfield: condenser position HDIC DIC - contrast: condenser position DIC Plan - NEOFLUAR 40X, NA 0.75 Ph 2 (air; n=1) Phase contrast: condenser position 2 Brightfield: condenser position HDIC DIC - contrast: condenser position DIC Plan - NEOFLUAR 63X, NA 1.25 Ph 2 (oil; n=1.518) Phase contrast: condenser position 2 Brightfield: condenser position HDIC DIC - contrast: condenser position DIC Plan - NEOFLUAR 100X, NA 1.30 (oil; n=1.518) Brightfield: condenser position HDIC DIC - contrast: condenser position DIC *NOTE: NA = numerical aperture; n = refractive index)