OUTLINE V2 - Concordia University
advertisement
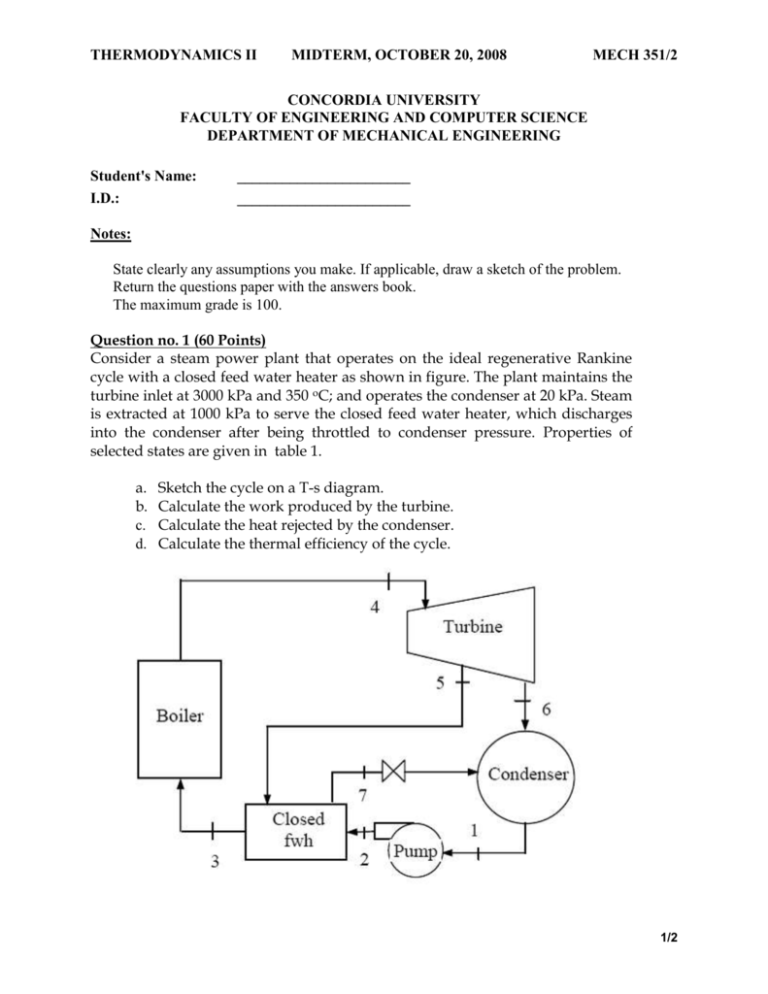
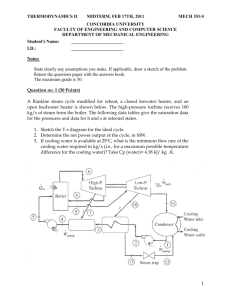
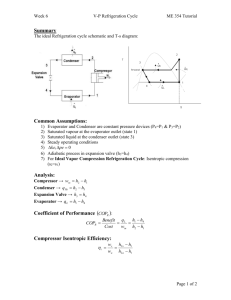
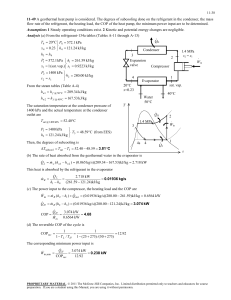
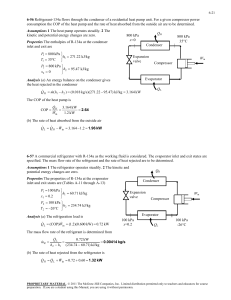
THERMODYNAMICS II MIDTERM, OCTOBER 20, 2008 MECH 351/2 CONCORDIA UNIVERSITY FACULTY OF ENGINEERING AND COMPUTER SCIENCE DEPARTMENT OF MECHANICAL ENGINEERING Student's Name: I.D.: _______________________ _______________________ Notes: State clearly any assumptions you make. If applicable, draw a sketch of the problem. Return the questions paper with the answers book. The maximum grade is 100. Question no. 1 (60 Points) Consider a steam power plant that operates on the ideal regenerative Rankine cycle with a closed feed water heater as shown in figure. The plant maintains the turbine inlet at 3000 kPa and 350 oC; and operates the condenser at 20 kPa. Steam is extracted at 1000 kPa to serve the closed feed water heater, which discharges into the condenser after being throttled to condenser pressure. Properties of selected states are given in table 1. a. b. c. d. Sketch the cycle on a T-s diagram. Calculate the work produced by the turbine. Calculate the heat rejected by the condenser. Calculate the thermal efficiency of the cycle. 1/2 THERMODYNAMICS II MIDTERM, OCTOBER 20, 2008 MECH 351/2 Table 1 Property h1 h2 h3 h4 s4 h5 h6 h7 Value 251.40 kJ/kg 254.45 kJ/kg 763.53 kJ/kg 3116.10 kJ/kg 6.745 kJ/kg.K Determine it 2311.10 kJ/kg Determine it Question no. 2 (40 Points) An air-standard Diesel cycle operates with a compression ratio of 16 and a cutoff ratio of 2. At the beginning of compression process the conditions are 37°C, 0.10 MPa, respectively. Assuming constant specific heats for air at room temperature (Cp = 1.005 kJ/kg.K; Cv = 0.718 kJ/kg.K and R=0.287 kJ/kg.K), determine: (a) (b) (c) (d) (e) The maximum temperature in the cycle. The pressure after the isentropic expansion. The net work per cycle and thermal efficiency. The mean effective pressure. The volume flow rate of air in m3/min, measured at conditions at the beginning of compression, needed to produce 200 KW. Isentropic relations T2 v 1 T1 s cte v2 k 1 T2 P 2 T1 s cte P1 P2 v 1 P1 s cte v2 C k p Cv k 1 k k 2/2