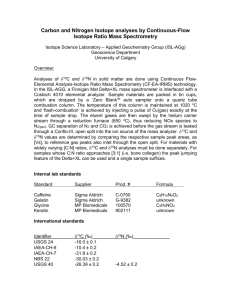
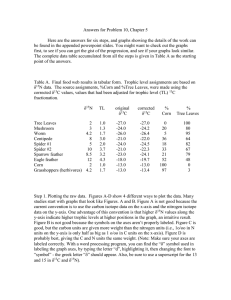
Chapter 2 figures.ppt
advertisement

Fig. 2.1. Linear relationships of H,C,N,O and S heavy isotope contents to d values. Large natural abundance d variations from 100 to +100o/oo correspond to only slight variations in percent heavy isotope, so that the effect of using d values is to greatly magnify the small natural differences found in nature. Also, the strong relationships shown between d values and “% heavy isotope” means that d values can be used to track heavy isotope dynamics in accounting and budget equations used later in this book for I Chi modeling. Lastly, the range shown here is for natural samples. Isotope can be purchased and added to natural systems, raising values to 1000o/oo and above. Outside the natural abundance range, the depicted linear relationships do not hold, and become increasingly curvilinear. Data used for the lines in these graphs were calculated from the definition of d and using standards listed in Table 2.1, with SMOW used as the standard for oxygen isotopes. The basic equation used for the calculations derives from the d definition as HAP = 100*(d + 1000)/[(d + 1000 + (1000/R STANDARD)] where % heavy isotope is atom % of the heavy isotope, or HAP. Calculations with this equation were modified for oxygen and sulfur that have more than 2 stable isotopes, assuming that the minor O and S isotopes were fractionated according to massdependent rules (Hulston and Thode 1965, Hoefs 2004; d17O = 0.515* d18O, d33S = 0.515*d34S and d36S = 1.9*d34S). Depicted best-fit lines reflect natural conditions and have r2 values of 1.0000. Equations for the lines are as follows: hydrogen (% 2H = 0.0000156*d2H + 0.0155726), carbon (%13C = 0.00109*d13C + 1.10559), nitrogen (%15N = 0.000365*d15N + 0.366295), oxygen (%18O = 0.000200*d18O + 0.200041) and sulfur (%34S = 0.00400*d34S + 4.19652). Fig. 2.2. Schematic of an isotope ratio mass spectrometer used to make isotope determinations. In the source region, gas molecules are ionized as they encounter electrons boiling off a hot filament. The charged ions are accelerated via electric fields through a stainless steel flight tube (not shown) maintained under vacuum. In the central magnetic field, charged ions are separated according to inertia, and dispersed towards collectors for automated counting by computers. Due to their small masses and consequent low inertia, the hydrogen ion beams are sharply bent by magnet focusing, while magnet focusing results in much more gradual bends in flight paths of the ion beam for gases with higher masses, especially CO 2, N2, O2 and SO2. Figure 2.3. There is a linear relationship between the three types of isotope notation (d, R and F) for natural samples in the 100 to +100o/oo d range. This example shows how R and F are related to d for carbon isotopes, d13C = [(RSAMPLE/RSTANDARD) - 1]*1000 where R = HF/LF = 13C/12C, HF = 13C, LF = 12C, the standard is a carbonate limestone (VPDB; see Table 2.1), and RSTANDARD = HF/LFSTANDARD = 0.011056/0.988944 = .011180. Fig. A (Technical Supplement A). Relationships between d values and atom %13C. Top: natural abundance range of d values that correspond to about 1.08 to 1.12%13C. Bottom: larger range of atom % values, 0-10%13C, corresponding to a larger range in d values. Note that no 13C (% 13C = 0) corresponds to -1000o/oo d value (see text for explanation). Isotope depleted samples thus have a lower limit (-1000o/oo), but isotope enriched samples have no upper limit, as evident in the next figures. Fig. B (Technical Supplement A). Relationships between d values and atom %13C, continued. Top: larger range of atom % values, 0-50%13C. Note the relationship between atom % and d starts to become visibly non-linear at higher enrichments. Bottom: largest range of atom % values 0-100%. At high enrichments >90% 13C, d and atom % are related in a very nonlinear fashion. Fig. C (Technical Supplement A). Nitrogen isotopes in N2 gas samples at different 15N enrichments. Modern mass spectrometers collect three isotopomers (isotope varieties) of N2 that have masses 28 (14N14N), 29 (15N14N and 14N15N) and 30 (15N15N). In natural samples, most N is 14N with only small amounts of 15N, so most N2 exists as mass 28 with minor amounts as mass 29 and very, very minor amounts as mass 30. However, this can change when scientists start adding 15N to samples, increasing the fraction that is 15N while simultaneously decreasing the amount that is 14N. Eventually, when there is no 14N, all N is present as mass 30. At intermediate mixtures of 14N and 15N, both masses 29 and 30 are important carriers of 15N and both these masses need to be measured and considered when keeping track of 15N amounts. Fig. D (Technical Supplement A). Nitrogen isotopes in N2 gas samples at different 15N enrichments, continued from previous Fig. 2.6. Top: In most usual d measurements, only the mass 28 (14N14N) and mass 29 (15N14N) isotopomers are considered, because the mass 30 (15N15N) contributions are very, very minor. But at higher 15N enrichments, considering only masses 28 and 29 will underestimate actual amounts of 15N, as shown in the previous Fig. 2.6. The problem is that much 15N is in mass 30 for enriched samples, and this is ignored in most d calculations. Bottom: Estimated error in d for enriched samples when mass 30 is ignored. Fig. E (Technical Supplement A). Correction factors (o/oo) for d values measured at relatively small 15N enrichments, <2000o/oo. For example, at 1% 15N (about 1750o/oo d15N), one needs to add about 8.8o/oo to the normal measured d value to obtain the correct d15N value, when the normal d value is calculated from the mass 28 and mass 29 ion beams without including the mass 30 ion beam.