Name: Date: Isotopes Worksheet 2 What is an isotope? How can
advertisement
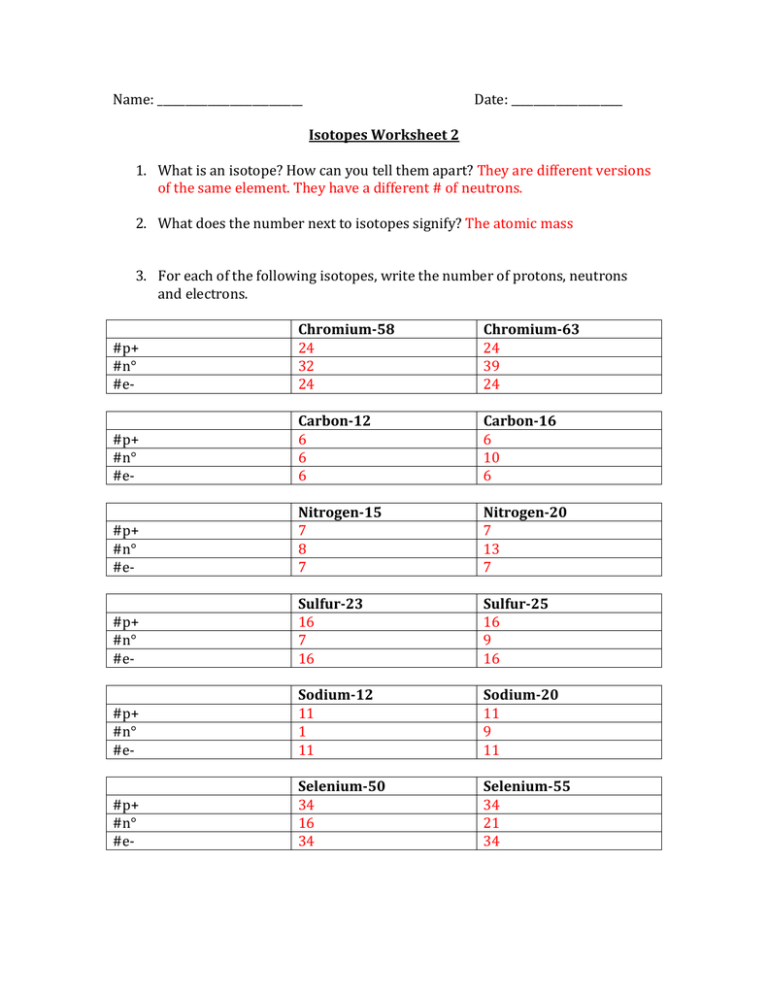
Name: __________________________ Date: ____________________ Isotopes Worksheet 2 1. What is an isotope? How can you tell them apart? They are different versions of the same element. They have a different # of neutrons. 2. What does the number next to isotopes signify? The atomic mass 3. For each of the following isotopes, write the number of protons, neutrons and electrons. #p+ #n° #e- Chromium-58 24 32 24 Chromium-63 24 39 24 #p+ #n° #e- Carbon-12 6 6 6 Carbon-16 6 10 6 #p+ #n° #e- Nitrogen-15 7 8 7 Nitrogen-20 7 13 7 #p+ #n° #e- Sulfur-23 16 7 16 Sulfur-25 16 9 16 #p+ #n° #e- Sodium-12 11 1 11 Sodium-20 11 9 11 #p+ #n° #e- Selenium-50 34 16 34 Selenium-55 34 21 34 4. Fill in the isotope names and any missing information, including isotope numbers from the chart. Use your periodic table and the information provided. #p+ #n° #e- Manganese-42 25 17 25 Manganese-40 25 15 25 #p+ #n° #e- Germanium-62 32 30 32 Germanium-64 32 32 32 #p+ #n° #e- Palladium-94 46 48 46 Palladium-97 46 51 46 Cesium-168 Cesium-166 #p+ #n° #eSymbol 17O8 131Xe54 207 82PB 23Na11 88Sr38 24Ne10 238 92U 55 113 55 Name of the element Oxygen-17 55 111 55 Atomic # Xenon-131 8 54 Lead-207 82 Sodium-23 11 Stronium-88 38 Neon-24 10 Uranium-238 92 #p+ #n° #e- Mass # 8 9 8 17 54 77 54 131 82 125 82 207 11 12 11 23 38 50 38 88 10 14 10 24 92 146 92 238





















