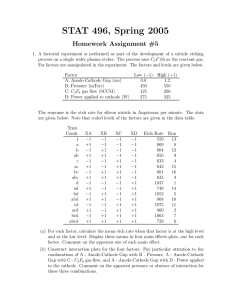
Name:________________________ Solutions Wet etching, introduction to vacuum technology
advertisement

Name:________________________ PHGN/CHEN/MLGN435/535 Pre-Lab #5 -- Solutions Topics: Wet etching, introduction to vacuum technology Reading: Campbell 11.1 p.297-303 [wet etching], 19.5 p. 608-615 [wet etching in MEMS processes], 10.1 p. 271-274 [vacuum conditions] Background: Wet etching has pretty much been abandoned, but has returned with MEMS devices and others that are still being researched. Vacuums are used extensively in plasma etching, evaporation, sputtering, etc. 1. In the next few weeks we will be wet etching your oxides in Buffered Oxide Etch (BOE). What are the reasons for adding the buffering agent to the HF? What is the typical etch rate of the BOE etch we use in the lab? Is this etch selective (explain why or why not)? Is it anisotropic (explain your answer)? We add the buffering agent for two reasons: the first is to maintain a constant HF concentration as the etch is used so the etch rate remains constant, the second is to control PH to minimize photoresist attack. Our BOE etches about 100nm (1000 angstroms) of thermally-grown oxide per minute. BOE is selective. It etches SiO2 must faster than Si. As a wet chemical etch, however, it if fairly isotropic, etching laterally and vertically at the same rate. 2. Campbell #11.2 (c) As a final component of this problem, is this etch a polishing etch or will it leave a rough surface? The etch composition is 20% acetic acid, 20% HF (49.2%) and 60% nitric acid (69.5%). This point is marked on both of the above graphs. a) From the left side we see the approximate etch rate is 35µm/min. If we want to etch a 700µm deep hole, it will take 700µm /35µm /min.=20min b) The etch rate could be reduced through: a. Etchant depletion, using up the chemicals in the solution. A solution would be to add additional chemicals as the etch proceeds. The most likely chemical to be depleted is HF due to its lower concentration. b. Gas bubbles could be forming at the surface blocking the chemicals from reaching the surface. A solution would be agitation. c. Diffusion of chemicals to the surface as they are consumed could be limiting the etch rate. Agitation would also help this. c) From the right side graph, we see it's a polishing etch. 3. A certain etch process has an anisotropy value of W A = 0.75. A perfect photoresist process defines a W = 1 µm wide line on an oxide layer that is Oxide 4000Å thick. Due to non-uniformity concerns the sample must be over-etched by 10% to make sure Silicon that all the oxide is removed. About how wide is the resulting oxide channel? (Assume the etched oxide sidewalls are vertical. In reality they would likely show some curvature.) If we want a 10% over etch, we need to etch for enough time that we would remove 4400 Å of oxide. The anisotropy is A=1-RL/RV = 0.75 which means RL/RV = 0.25. Therefore DL/DV = 0.25 where DL is the amount of material removed laterally and DV is the amount removed vertically, so DL=4400 Å x 0.25 = 1100 Å. Since this lateral etch will appear on both sides of the line, the line width will be 1µm+2x0.11µm= 1.22µm. 4. Campbell #19.12 (oxides revisited!) From Fig. 19.14, for 42% KOH etch at 80C the ratio of the etch rate for Si over SiO2 is about 150. If we etch 400µm of Si, then in the same time we will etch 400µm/150 = 2.67µm of SiO2. If we want 2000 Å of SiO2 to remain, then we need to start with 2.87µm. Returning to the Deal-Grove model in Chapter 4 and table 4.1, for wet oxide at 1100C, A=0.11µm and B=0.510µm2/hr. The initial oxide thickness is 25 Å. From Eq. 4.12 we get τ =((.0025µm)2 + 0.11µm x .0025µm)/0.510µm/hr = 5.5x10-4hr which is essentially negligible as might be expected. Remember, τ is the time offset associated with preexisting oxide. Now, finally, from Eq. 4.11 we have (2.87µm) 2+ 0.11 x2.87µm2=0.510(t+τ) which gives t+τ ~ t = 16.8hr. So we must grow the wet oxide for nearly 17 hr. This gives you some idea of why deposited oxides which can be prepared in thick layers much more rapidly can be important for mask steps like these. 5. What is the approximate mean free path, at room temperature, of molecules in the RIE at 300mtorr? Of molecules in the metal evaporator at 3x10-6 torr? What do these answers tell you about the likelihood of molecule-molecule collisions in these two systems? Be careful about units here, and make sure your answers make sense. Eq. 10.9 gives λ=kT/(√2πd2P). d is a cross section. We will use the book’s value of 3 Å. At 300K we get λ=1.38x10-23J/K x 300K/(√2π (3x10-10m) 2P)=0.01035/P where P is in Pascal's. For P in torr, λ=0.01035/(Ptorrx133.3)=7.77x10-5/Ptorr where the answer is in meters. For Ptorr=300mtorr, λ=260µm, while for Ptorr=3x10-6torr, λ=26m. Notice for the higher pressure, collisions occur on a much shorter distance than typical dimensions in an RIE, while in the latter case, molecules are more likely to hit the walls of a vacuum system, then they are to hit each other. These are both important length scales for the respective operations we do in these systems, etching in one case and deposition in the other.