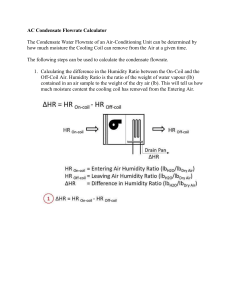
volume flow rate of the mixture are to be determined. 4
advertisement

14-62 14-105 Two airstreams are mixed steadily. The specific humidity, the relative humidity, the dry-bulb temperature, and the volume flow rate of the mixture are to be determined. Assumptions 1 Steady operating conditions exist 2 Dry air and water vapor are ideal gases. 3 The kinetic and potential energy changes are negligible. 4 The mixing section is adiabatic. Properties Properties of each inlet stream are determined from the psychrometric chart (Fig. A-31) to be h1 62.7 kJ/kg dry air 1 0.0119 kg H 2 O/kg dry air v 1 0.882 m 3 /kg dry air 1 and 32C 40% 20 m3/min h2 31.9 kJ/kg dry air P = 1 atm AIR 2 0.0079 kg H 2 O/kg dry air v 2 0.819 m 3 /kg dry air Analysis The mass flow rate of dry air in each stream is m a1 V1 20 m 3 / min 22.7 kg/min v 1 0.882 m 3 / kg dry air m a 2 V2 25 m 3 / min 30.5 kg/min v 2 0.819 m 3 / kg dry air 2 3 3 3 T3 25 m3/min 12C 90% From the conservation of mass, a3 m a1 m a 2 ( 22.7 30.5) kg / min 53.2 kg / min m The specific humidity and the enthalpy of the mixture can be determined from Eqs. 14-24, which are obtained by combining the conservation of mass and energy equations for the adiabatic mixing of two streams: a1 2 3 h2 h3 m a 2 3 1 h3 h1 m 22.7 0.0079 3 319 . h3 30.5 3 0.0119 h3 62.7 which yields, 3 0.0096 kg H 2O / kg dry air h3 45.0 kJ / kg dry air These two properties fix the state of the mixture. Other properties of the mixture are determined from the psychrometric chart: T3 20.6C 3 63.4% v 3 0.845 m 3 /kg dry air Finally, the volume flow rate of the mixture is determined from V3 m a 3v 3 (53.2 kg/min)(0.845 m 3 / kg) 45.0 m 3 /min PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.