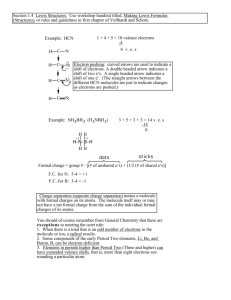
Name:_____________ Chemistry 112 Final Exam
advertisement

Name:_____________ Chemistry 112 Final Exam Section I . New Material Each questions worth 25 points- You may NOT skip any questions from this section. 1. In the ground state of Cd How many electrons occupy atomic orbitals with n=3? How many electrons occupy orbitals where R=2 How many electrons occupy orbitals where R=1 & mR = -1 How many electrons have an ms of +1/2 How many electrons occupy an orbital where n=5 and & mR = -4 2. Write electron configurations fr the following atoms or ions: N S2- Cu An excited state of Na Nd 2 3. Estimate ÄHRXN for the reaction K(s) + ½ Cl2(g) 6 KCl(s) using the following data: Lattice energy -695 kJ/mol Ionization Energy for K 419 kJ/mol Electron affinity of Cl -349 kJ/mol Bond Energy of Cl2 239 kJ/mol Enthalpy of sublimation for K 64 kJ/mol 4. For each of the following molecules or ions: 1. Provide a Lewis structure. 2. Give the arrangement of electrons in the molecule. 3. Give the molecular configuration of the molecule. 4. Tell if the molecule contains polar bonds. 5. Tell if the molecule is polar. SF5 PO43+ AlCl3 NF3 3 Section II Old Material You may do any 4 questions. (All questions worth 25 points. - If you do more than 4 questions, I will throw out the worst one(s).)