Half-Life Notes Isotope Half Life
advertisement

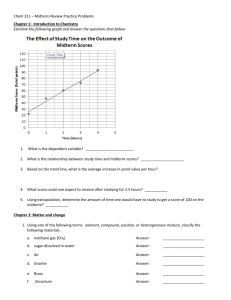
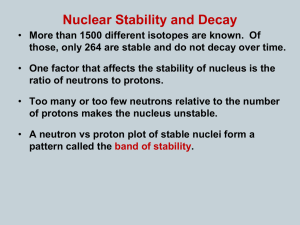
Half-Life Notes I. Isotope- an element with a different number of neutrons but same number of protons a. Example Hydrogen may have 0 Neutrons, 1 Neutron or 2 Neutrons II. Half Lifea. Measures the decay of atoms over time b. Often the loss of neutrons from one isotope forming another isotope. c. The time it takes for ½ of an original amount to decay d. Questions: Answer the question below with help from your table. 1. If a sample has a half-life of 1 day, how much of sample will be left after… a. 1 day? b. 2 days? c. 3 days? Answers: a. ½ b. ¼ c. 1/8 2. 100g of element X has a half-life of 10 yrs. How much will be left after… a. 10 yrs.? b. 100 yrs.? Answers: a. 50 g b. .0977 g e. Real World Application- Carbon dating i. Carbon 14 decays and over time becomes Carbon 12 ii. Carbon 14 has a half life of 5730 yrs iii. By measuring how much Carbon 14 is in an object you can tell how old it is.