Biology Chapter #2
advertisement

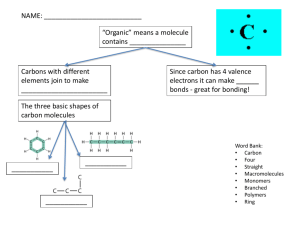
The Chemistry of Life The Nature of Matter Atoms All things are made of matter Atom -basic unit of all matter Normally it is electrically neutral (no charge) Parts of the atom Protons Neutrons Electrons The Nature of Matter Protons Protons + charged particle In the center of the atom-nucleus Heavy The Nature of Matter Neutrons Neutrons Neutrally charged particle In the nucleus Heavy The Nature of Matter Electrons Electrons - charged particle In constant motion in orbitals surrounding the nucleus Light The Nature of Matter Elements Element-pure substance that consists of just one type of atom Atomic Number= # of protons=#electrons 114 in the periodic table – Only about 2 dozen commonly found 6 in living things Atoms have a 1 or 2 letter symbol C = carbon N = nitrogen H = hydrogen O = oxygen, etc. C Carbon 12.011 Atomic Mass= # of protons + # of neutrons Where is Sodium? What is its atomic number? The Nature of Matter Isotopes •Isotopes--atoms of same element w/ a different # of neutrons Isotopes of Carbon Nonradioactive carbon-12 Nonradioactive carbon-13 6 electrons 6 protons 6 neutrons 6 electrons 6 protons 7 neutrons Radioactive carbon-14 6 electrons 6 protons 8 neutrons The Nature of Matter Radioactive Isotopes Isotope with unstable nucleus due to extra neutrons Break down at a constant rate Give off dangerous radiation Have uses too C-14 dating- determines ages of rocks by analyzing isotopes found in them Cancer treatment The Nature of Matter Compounds In nature, most elements are found combined with other elements in nature. A chemical compound is a substance formed by the chemical combination of two or more elements in definite proportions. The atoms in compounds are held together by chemical bonds. The Nature of Matter Bonding Bonding -for some atoms to be stable they must gain, lose, or share electrons with another atom. 2 types of bonds -Ionic -Covalent The Nature of Matter Ionic Bonds Ionic bonds - electrons transferred from one atom to another Valence electrons- electrons in the last energy level of an atom Formation of ions-negatively or positively charged atoms The oppositely charged ions attract, forming the ionic bond NaCl has ionic bonds Ionic Bonding Sodium atom (Na) Chlorine atom (Cl) Sodium ion (Na+) Chloride ion (Cl-) Transfer of electron Protons +11 Electrons -11 Charge 0 Protons +17 Electrons -17 Charge 0 Protons +11 Electrons -10 Charge +1 Protons +17 Electrons -18 Charge -1 The Nature of Matter Covalent Bonds Covalent bonds --sharing electrons Stronger than ionic Molecule--formed when atoms are joined in a covalent bond Properties of Water Water covers ¾ of Earth’s surface Most abundant molecule in living things Universal solvent…dissolver of many substances found on Earth Connects all parts of the world with others. Properties of Water Polar Molecule Water has covalent bonds Water bonds are polar- unequal sharing of electrons. Oxygen “pulls” harder on negative electrons than H—gives O slight negative charge. **Water has polar, covalent bonds Properties of Water Hydrogen Bonds Polarity causes water molecules to attract each other like magnets H (+) attracts O (-) forming a hydrogen bond Gives water special properties Properties of Water Hydrogen Bonds Hydrogen bonds are not as strong as covalent or ionic Waters ability to form multiple hydrogen bonds is responsible for many of it’s special properties Cohesion – is an attraction between molecules of the same substance Adhesion – is an attraction between molecules of different substances Properties of Water Solutions Solutions occur when one substance is dissolved in another. Solute -gets dissolved Solvent -does the dissolving. Saltwater What is the pH scale? The pH scale measures how acidic or basic a solution is. The pH scale The pH scale is the concentration of hydrogen ions in a given substance. Identifying Acids and Bases Acids have a ph from 0-6 Lower pH value indicates a stronger acid. Bases have a pH from 8-14 Higher pH value indicates a stronger base. OH Definitions of Acids and Bases An acid is a substance that breaks into H ions in an aqueous solution. A Base (alkaline) is a substance that breaks into OH ions in an aqueous solution. Note: aqueous solution is any solution where H 2 O is the solvent. Did we Miss something?? What happens when the pH of a substance is 7? Ans: A pH level of 7 indicates a Neutral Substance i.e: Water! Test Your Knowledge What is the range of an ACID on the pH scale? Ans: 0-6 What is the range of a BASE and what is another name for a BASE? Ans: 8-14, Alkaline Characteristics Of Acids Acids can be characterized by: 1. A sour taste. 2. It turns blue litmus paper red 3. It tastes sour. Try drinking lemon juice (citric acid) Characteristics of Bases A Base is characterized by: 1. A bitter taste. (Milk of Magnesia) 2. It feels slippery. (Soapy Water) 3. It turns Red Litmus Blue. Why Learn about Acids & Bases? What do you think is the pH level of miami dade county tap water? The pH of a swimming pool must be checked periodically. Why? Is it important for Lakes & Rivers to maintain a certain pH? Today’s Experiment Test the pH of Pepsi, tap water, and drain cleaner GOOD LUCK!!! Properties of Water Buffers Human homeostasis-human blood must be b/t pH 6.77.5 Body uses buffers, which are weak acids or bases, to neutralize sharp changes in blood pH Macromolecules copyright cmassengale 32 Organic Compounds Compounds that contain CARBON are called organic. Macromolecules are large organic molecules. copyright cmassengale 33 Carbon (C) Carbon has 4 electrons in outer shell. Carbon can form covalent bonds with as many as 4 other atoms (elements). Usually with C, H, O or N. Example: CH4(methane) copyright cmassengale 34 Macromolecules Large organic molecules. Also called POLYMERS. Made up of smaller “building blocks” called MONOMERS. Examples: 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleiccopyright acids (DNA and RNA) cmassengale 35 Question: How Are Macromolecules Formed? copyright cmassengale 36 Answer: Dehydration Synthesis Also called “condensation reaction” Forms polymers by combining monomers by “removing water”. http://nhscience.lonestar.edu/biol/dehydrat/dehydrat.html HO H HO H H2O HO H copyright cmassengale 37 Question: How are Macromolecules separated or digested? copyright cmassengale 38 Answer: Hydrolysis Separates monomers by “adding water” HO H H2O HO H copyright cmassengale HO H 39 Carbohydrates copyright cmassengale 40 Carbohydrates Small sugar molecules to large sugar molecules. Examples: A. monosaccharide B. disaccharide C. polysaccharide copyright cmassengale 41 Carbohydrates Monosaccharide: one sugar unit Examples: glucose glucose (C6H12O6) deoxyribose ribose Fructose Galactose copyright cmassengale 42 Carbohydrates Disaccharide: two sugar unit Examples: Sucrose (glucose+fructose) Lactose (glucose+galactose) Maltose (glucose+glucose) glucose glucose copyright cmassengale 43 Carbohydrates Polysaccharide: many sugar units Examples: starch (bread, potatoes) glycogen (beef muscle) cellulose (lettuce, corn) glucose glucose glucose glucose cellulose glucose glucose glucose copyright cmassengale glucose 44 Lipids copyright cmassengale 45 Lipids General term for compounds which are not soluble in water. Lipids are soluble in hydrophobic solvents. Remember: “stores the most energy” Examples: 1. Fats 2. Phospholipids 3. Oils 4. Waxes 5. Steroid hormones cmassengale 46 6. copyright Triglycerides Lipids Six functions of lipids: 1. Long term energy storage 2. Protection against heat loss (insulation) 3. Protection against physical shock 4. Protection against water loss 5. Chemical messengers (hormones) 6. Major component of membranes (phospholipids) copyright cmassengale 47 Lipids Triglycerides: composed of 1 glycerol and 3 fatty acids. H O H-C----O C-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH3 O H-C----O C-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH3 O fatty acids H-C----O C-CH -CH -CH -CH 2 2 2 H glycerol copyright cmassengale 48 Fatty Acids There are two kinds of fatty acids you may see these on food labels: 1. Saturated fatty acids: no double bonds (bad) O saturated C-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH3 2. Unsaturated fatty acids: double bonds (good) O unsaturated C-CH2-CH2-CH2-CH copyright cmassengale 49 Proteins copyright cmassengale 50 Proteins (Polypeptides) Amino acids (20 different kinds of aa) bonded together by peptide bonds (polypeptides). Six functions of proteins: 1. Storage: albumin (egg white) 2. Transport: hemoglobin 3. Regulatory: hormones 4. Movement: muscles 5. Structural: membranes, hair, nails 6. Enzymes: cellular reactions copyright cmassengale 51 Proteins (Polypeptides) Four levels of protein structure: A.Primary Structure B. Secondary Structure C. Tertiary Structure D.Quaternary Structure copyright cmassengale 52 Primary Structure Amino acids bonded together by peptide bonds (straight chains) Amino Acids (aa) aa1 aa2 aa3 aa4 aa5 aa6 Peptide Bonds copyright cmassengale 53 Secondary Structure 3-dimensional folding arrangement of a primary structure into coils and pleats held together by hydrogen bonds. Two examples: Alpha Helix Beta Pleated Sheet Hydrogen Bonds copyright cmassengale 54 Tertiary Structure Secondary structures bent and folded into a more complex 3-D arrangement of linked polypeptides Bonds: H-bonds, ionic, disulfide bridges (S-S) Call a “subunit”. Alpha Helix Beta Pleated Sheet copyright cmassengale 55 Quaternary Structure Composed of 2 or more “subunits” Globular in shape Form in Aqueous environments Example: enzymes (hemoglobin) subunits copyright cmassengale 56 Nucleic Acids copyright cmassengale 57 Nucleic acids Two types: a. Deoxyribonucleic acid (DNAdouble helix) b. Ribonucleic acid (RNA-single strand) Nucleic acids are composed of long chains of nucleotides linked by dehydration synthesis. copyright cmassengale 58 Nucleic acids Nucleotides include: phosphate group pentose sugar (5-carbon) nitrogenous bases: adenine (A) thymine (T) DNA only uracil (U) RNA only cytosine (C) guanine (G) copyright cmassengale 59 Nucleotide Phosphate Group O O=P-O O 5 CH2 O N C1 C4 Nitrogenous base (A, G, C, or T) Sugar (deoxyribose) C3copyright cmassengale C2 60 DNA double helix O 5 3 3 O P 5 O C G 1 P 5 3 2 4 4 2 3 1 P T 5 A P 3 O O P 5 O 3 copyright cmassengale 5 P 61 Organic Macromolecules Carbohydrates Structure: subunit = sugars Carbs are either Sugars = monosaccharides sucrose, glucose, fructose short chains OR Starches = polysaccharides sugars hooked together pasta, cellulose (plant cell walls) long chains MONOSACCHARIDES POLYSACCHARIDE Organic Macromolecules Carbohydrates Function Main source of energy for living things Converted to ATP—gasoline for cells Monosaccharides = immediate E Polysaccharides = longer term E Plants store carbs as cellulose—gives their cells strength Organic Macromolecules Lipids Structure: subunit = fatty acids and glycerol C and H Fats, oils, waxes are lipids Saturated and Unsaturated fats Hydrophobic—”water-fearing”-not dissolvable in water FATTY ACIDS AND GLYCEROL LIPID Organic Macromolecules Lipids Function Store energy for use later Why should I care? Hormones Cell membranes Waterproof covering (skin) Organic Macromolecules Nucleic Acids Structure: subunit = nucleotides Nucleotides: 5 C sugar Phosphate group Nitrogenous base DNA and RNA NUCLEOTIDES NUCLEIC ACID Organic Macromolecules Nucleic Acids Function: Store and transmit hereditary (genetic) info Organic Macromolecules Proteins Structure: subunit = amino acids C, N, O, and H 20 different amino acids form 1000’s of different proteins AMINO ACIDS PROTEINS Organic Macromolecules Proteins Function Enzymes-proteins that control chemical reactions Form bones and muscles Forms hair and nails (keratin) Part of cell membrane Steak, eggs, nuts, cheese PROTEINS ARE NOT SOURCES OF ENERGY! ORGANIC MACROMOLECULES C-C BONDS LIPIDS Subunit: Glycerol and fatty acids Fats, oils Waxes Hydrophobic Cell Membrane Hormones Energy storage NUCLEIC ACIDS Subunit: Nucleotides DNA and RNA Store and carry genetic information CARBOHYDRATES Subunit: Simple sugars (monosaccharides) Polysaccharides are starches like pasta and potatoes 1C: 2H: 1O Both for energy Cellulose-cell Walls in plants PROTEINS Subunit: Amino acids Steak, eggs peanuts Help build muscle, hair and nails Act as enzymes Cell membrane Chemical Reactions and Enzymes Chemical Reactions Everything occurring in an organism based on chemical reactions- process that changes one set of chemicals into another set Slow (rusting) or fast (burning) Breaking and remaking of bonds Reactants-products Chemical Reactions and Enzymes Chemical Reactions Reactant(s) Product(s) carbon + oxygen carbon dioxide + energy CO2 + energy C + O2 O C O C O black solid colorless gas colorless gas O + energy Chemical Reactions and Enzymes Energy in Chemical Reactions Reactions involve changes in energy Some reactions release energy and some have to absorb it ATP- form of energy absorbed or released when bonds are made or broken We get our ATP and organic macromolecules for body processes from food. Plants get it through? Chemical Reactions and Enzymes Some reactions are too slow or need lots of activation energy (ATP) to occur. Enzymes are proteins that decrease the activation energy needed for these types of reactions to occur. They lower the energy “hill” making these reactions easier and faster Digestion, nervous system signals, etc. all require enzymes. Activation energy Reactants Products Chemical Reactions and Enzymes Enzymes Enzyme activity regulated by many variables pH Temperature Enzyme concentration PRODUCTS REACTANTS