Lesson 9 & 10 - Catalysts at work
advertisement

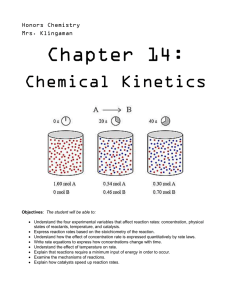
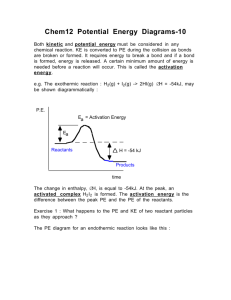
carbon dioxide hydrochloric acid calcium carbonate (marble) After completing this topic you should be able to : • Describe the mechanism of heterogeneous catalysis • Learn how a catalyst speeds up reaction rate by lowering the activation energy, and how to represent this on a potential energy diagram. Catalysts at Work Heterogeneous When the catalyst and reactants are in different states you have ‘Heterogeneous Catalysis’. They work by the adsorption of reactant molecules. E.g. Ostwald Process (Pt) for making nitric acid and the Haber Process (Fe) for making ammonia and the Contact Process (Pt) for making Sulphuric Acid. Homogeneous When the catalyst and reactants are in the same state you have ‘Homogeneous Catalysis’. E.g. making ethanoic acid from methanol and CO using a soluble iridium complex. Enzymes are biological catalysts, and are protein molecules that work by homogeneous catalysis. E.g. invertase and lactase. Enzymes are used in many industrial processes How a heterogenous catalyst works Heterogenous Catalysis are thought to work in three stages... Adsorption Reaction Desorption Higher Chemistry Eric Alan and John Harris How a heterogenous catalyst works For an explanation of what happens click on the numbers in turn, starting with How a heterogenous catalyst works Adsorption (STEP 1) Incoming species lands on an active site and forms bonds with the catalyst. It may use some of the bonding electrons in the molecules thus weakening them and making a subsequent reaction easier. How a heterogenous catalyst works Adsorption (STEP 1) Incoming species lands on an active site and forms bonds with the catalyst. It may use some of the bonding electrons in the molecules thus weakening them and making a subsequent reaction easier. Reaction (STEPS 2 and 3) Adsorbed gases may be held on the surface in just the right orientation for a reaction to occur. This increases the chances of favourable collisions taking place. How a heterogenous catalyst works Adsorption (STEP 1) Incoming species lands on an active site and forms bonds with the catalyst. It may use some of the bonding electrons in the molecules thus weakening them and making a subsequent reaction easier. Reaction (STEPS 2 and 3) Adsorbed gases may be held on the surface in just the right orientation for a reaction to occur. This increases the chances of favourable collisions taking place. Desorption (STEP 4) There is a re-arrangement of electrons and the products are then released from the active sites Examples of heterogenous catalysts Format Metals Ni, Pt Fe Rh, Pd hydrogenation reactions Haber Process catalytic converters Oxides Al2O3 V2O5 dehydration reactions Contact Process FINELY DIVIDED increases the surface area provides more collision sites IN A SUPPORT MEDIUM maximises surface area and reduces costs How a heterogenous catalyst works In some cases the choice of catalyst can influence the products Ethanol undergoes different reactions depending on the metal used as the catalyst. The distance between active sites and their similarity with the length of bonds determines the method of adsorption and affects which bonds are weakened. Copper Dehydrogenation (oxidation) C2H5OH ——> CH3CHO + H2 Alumina Dehydration C2H5OH ——> C2H4 + H2O How a heterogenous catalyst works Poisoning Impurities in a reaction mixture can also adsorb onto the surface of a catalyst thus removing potential sites for gas molecules and decreasing efficiency. expensive because... the catalyst has to replaced the process has to be shut down examples Sulphur Lead Haber process catalytic converters in cars Investigating Catalysis The problem Transition metals and their compounds are said to be effective catalysts You are asked to link the transition metal compounds to their ability to act as a catalyst for the decomposition of hydrogen peroxide Materials available hydrogen peroxide solution (5-10 vol) cobalt(II) chloride (aq) copper(II) sulphate (aq) manganese(II) chloride (aq) nickel(II) nitrate (aq) bench dilute sodium hydroxide (approximately 1 mol l-1). Decomposition of hydrogen peroxide Hydrogen Peroxide → water + oxygen 2H2O2 → 2H2O + O2 What will you see during the reaction? What is the test for oxygen? Testing the catalysts Collect 5 test tubes in a rack To each add 5 cm3 of hydrogen peroxide Add 3 cm3 of a different catalyst to 4 test tubes, leaving the last one as a control Note the results Which type of catalysis? Did the experiment involve heterogeneous or homogeneous catalysis? Which catalyst was the most effective? Changing the solubility of the catalysts The transition metal ion in the compound has the catalytic effect. The ion can be found in solution (aqueous) or in a solid state. How could you use the chemicals provided to create transition metal ions in the solid state i.e. a heterogeneous catalyst? Investigate the effect of changing the state of the catalysts Which was the best catalyst overall? The students should find that none of the substances on their own is an effective catalyst but that the solid precipitates made by mixing any of the transition metal solutions with sodium hydroxide solution are good catalysts, ie this is heterogeneous catalysis. You may have to join in the discussion to ensure that the students realise this. An Example of a homogenous catalyst Dissolve 4 spatulas full of potassium sodium tartrate in a small beaker with about 2.5 cm depth of water. Repeat with another beaker (the control) To one beaker, add enough 10% cobalt chloride solution (catalyst) for the solution to be pink. Add 10 ml hydrogen peroxide to the beakers. Heat the control to around 60 - 80 oC and note observations Now heat the one with the catalyst and note observations An Example of a homogenous catalyst Higher Chemistry Eric Alan and John Harris This is an impressive demonstration of how a catalyst is involved in the progress of a reaction. Students can add another 10 cm3 of the hydrogen peroxide solution and if there is any potassium sodium tartrate remaining they will see a similar reaction. The reaction is an oxidation of the tartrate ion (proper name is 2, 3dihydroxybutandioate ion) to carbon dioxide gas and the methanoate ion. Hydrogen peroxide oxidises the tartrate ion very slowly if there is no catalyst, even at elevated temperatures. Cobalt(ll) ions are pink. The hydrogen peroxide initially oxidises the cobalt(II), Co2+, to cobalt(lll), Co3+, which is green. The cobalt(III) bonds to the tartrate ion, allowing the oxidation to take place. The CO3+ is then reduced back to CO2+ and the pink colour returns. The cobalt catalyst provides an alternative route for the reaction to occur. This alternative route has a lower activation energy and the reaction proceeds much more quickly. Catalysts and Potential energy diagrams Catalysts work by providing… “AN ALTERNATIVE REACTION PATHWAY WHICH HAS A LOWER ACTIVATION ENERGY” Visualising activated complex Potential energy graphs and catalysts Uncatalysed 75 - 60 50 - Reactants Catalysed P.E. Products 25 - Reaction path Potential energy graphs and catalysts Activation energy Ea for the forward uncatalysed reaction 75 - 60 - Activation energy Eafor the forward catalysed reaction Reactants 50 - P.E. Products 25 - Reaction path Catalysts lower the activation energy needed for a successful collision. Potential energy graphs and catalysts Activation energy Ea for the reverse uncatalysed reaction reaction 75 - Activation energy Ea for the reverse catalysed reaction 60 50 - Reactants P.E. Products 25 - Reaction path Catalysts lower the activation energy needed for a successful collision. Potential energy graphs and catalysts 75 - Catalysts lower the activation energy needed for a successful collision. 60 50 - Reactants P.E. Products 25 - Reaction path ∆H Activation energy Effect of catalyst – forward reaction No change Lowered Effect of catalyst – reverse reaction No change Lowered Catalysts A catalyst speeds up the reaction by lowering the activation energy. A catalyst does not effect the enthalpy change for a reaction A catalyst speeds up the reaction in both directions and therefore does not alter the position of equilibrium or the yield of product, but does decrease the time taken to reach equilibrium. Energy distribution and catalysts Ea No of Collisions with a given K.E. Un-catalysed reaction Kinetic energy Ea Total number of collisions (area under the graph) with sufficient K.E. energy to create new products. Catalysed reaction Ea is reduced