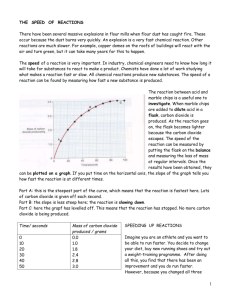
4.5 Catalysts
4.5 Catalysts
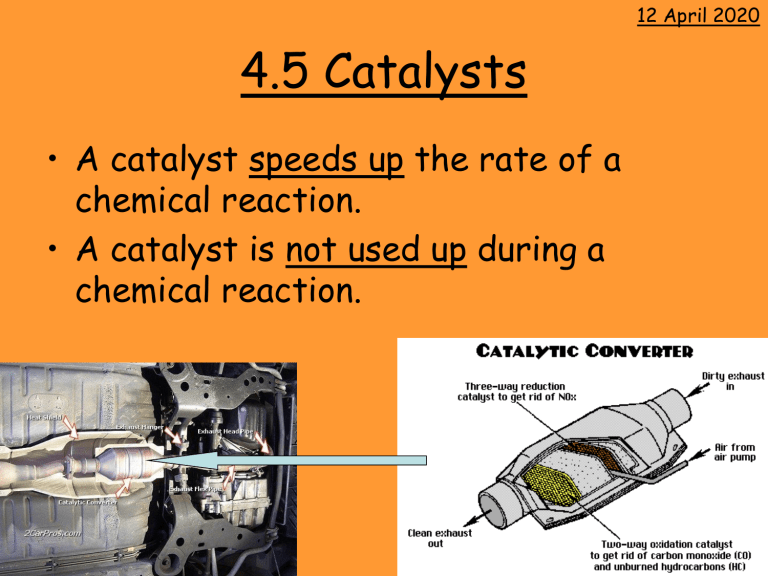
• A catalyst speeds up the rate of a chemical reaction.
• A catalyst is not used up during a chemical reaction.
12 April 2020
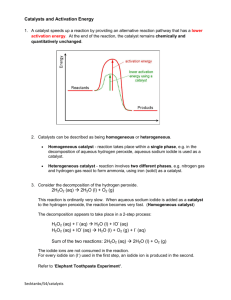
Decomposition of Hydrogen Peroxide
• Hydrogen peroxide decomposes into water and oxygen.
Hydrogen peroxide
water + oxygen
2H
2
O
2
(aq)
2H
2
O(l) + O
2
(g)
Oxygen gas is given off and can be measured using a gas syringe or a balance.
The reaction is catalysed by a wide range of solids.
Remember the catalyst NEVER produces more product - just quicker
Comparing catalysts
1. Measure out 25 cm 3 of hydrogen peroxide and put it into the conical flask.
2. Finely chop some celery and put into the flask.
3. Quickly connect the bung to the gas syringe and note the volume of gas produced every ten seconds for two minutes.
4. Repeat with chopped liver .
5. Repeat with manganese dioxide .
Results
Time
(seconds)
40
50
60
70
80
0
10
20
30
90
100
110
120
Gas produced with celery (cm 3 )
Excel sheet available – click on Results
Gas produced with liver
(cm 3 )
Gas produced with manganese dioxide
(cm 3 )
Graph
• Draw a graph of your results.
• What type of graph should it be?
• Remember to include
– Title
– Labels for each axis
– Units for each axis.
Which was the best catalyst?
Celery Liver Manganese (IV) oxide
4.5 Catalysts
12 April 2020
True or false?
How to play:
• Put your hand up for true, leave your hand down for false.
• Keep track of your score.
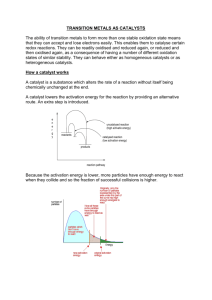
A catalyst is a substance that speeds up a chemical reaction but is not used up in the reaction.
TRUE
Most of the catalysts used in industry are acids.
FALSE – transition metals are mainly used.
The catalyst used in the Haber process is iron.
TRUE
The decomposition of hydrogen peroxide can be speeded up by adding a catalyst.
TRUE
Catalysts can become poisoned by impurities in the reaction so they need to be replaced.
TRUE
Catalysts work by increasing the number of collisions between reactant particles.
FALSE – they lower the energy needed to react.
Catalysts are usually cheap as they are readily available.
FALSE – they are expensive as they are made of precious metals.
There is a catalyst for every reaction.
FALSE – not every reaction has a suitable catalyst.
What is your score out of 8…?
Try to beat your score next time or get 100% again!
4.5 Catalysts
• A catalyst speeds up the rate of a chemical reaction.
• A catalyst is not used up during a chemical reaction.
12 April 2020
Homework: Find out what the catalyst materials used in cars are.