Scientific abstract
advertisement

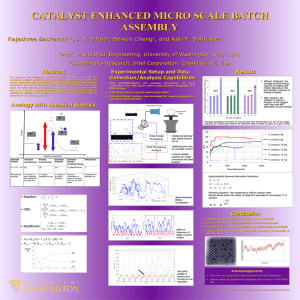
Abstract This thesis describes the synthesis of differently functionalized Cinchona alkaloids. These alkaloids can be applied in organocatalysis as bifunctional catalysts. Nowadays the most interest is in C9- and C6’-functionalized alkaloids. The C9-functionalized catalysts are well explored and the C6’-position is an emerging type of catalyst. This thesis will deal with two relatively new functional groups at the C6’-position, sulfonamide and squaramide. Hiemstra et al. have reported a thiourea catalyst. Deng et al. proved that it performs well in the addition of thiols to α,β-unsaturated N-acylated oxazolidin-2-one obtaining good optical purity. Several C6’-sulfonamide substituted catalysts are synthesized. The C6’-functionalized pmethoxysulfonamide synthesized catalyst proves to perform even better in a similar reaction. Remarkable however is that the sulfonamide catalyst provides the opposite enantiomer compared to the thiourea catalyst. The synthesis of squaramide catalysts was not successful, although a squaramide catalyst with a substituted 3,5-bis-trifluoromethylphenylgroup has been synthesized in our group. Other catalysts with C6’ functional groups are, urea and benzimidazool, both with two trifluoromethyl groups attached on the aromatic ring. Comparing these catalysts with each other no uniform trend could be detected. The acidity of the hydrogen bonding proton influenced the reaction positively comparing urea with thiourea, but in sulfonamide catalysis the more acidic catalysts provided poorer results.