MLAB 1227- Coagulation Keri Brophy
advertisement

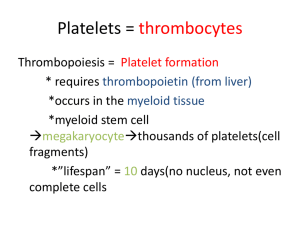
MLAB 1227- Coagulation Keri Brophy-Martinez Thrombophilia Thrombosis Imbalances between clotting activity and fibrinolytic processes Causes increased tendency to form thrombi Involves the naturally occurring inhibitors of coagulation More than one hemostatic defect or abnormality increases risk Procoagulant Factors F i b r i n o g e n Regulatory Factors Fibrin Balance of Hemostasis *Balance of bleeding (hemorrhaging) and clotting (thrombosis) *Imbalance in one direction can lead to: bleeding : hypocoagulable state OR thrombosis: hypercoagulable state Terms Hypercoagulation: more clotting activity than normal Thrombosis: inappropriate formation of platelet and/or fibrin clots that obstruct blood vessel Thrombus: stationary fibrin mass consisting of fibrin, platelets and trapped cells Embolus: piece of thrombotic material that moves Embolism: obstruction in circulatory system caused by embolus Blood Clot: a mass that forms extravascularly, either in vitro or in tissue Terms con’t Plaque: consists of lipids, fibrous connective tissue, macrophages and smooth muscle cells Thrombophlebitis: thrombus of superficial veins of legs; self-limiting and benign Deep vein thrombosis: involvement of deep veins of legs (iliac, femoral) Thrombophilia: any disorder associated with an increased tendency to cause thrombosis Ischemia: Local obstruction of a blood vessel by a thrombus, resulting in loss of blood supply Thrombus Formation Two types ◦ Arterial—white thrombi ◦ Venous—red thrombi Arterial Thrombus Formation Occurs when activation of blood coagulation exceeds ability of the anticoagulant/inhibitors and fibrinolytic system to prevent the formation of fibrin. White thrombi composed of platelets, fibrin and a few WBC’s and RBC’s Form at areas where the flow has been disturbed via damage to endothelium, especially atherosclerotic plaques Arterial Thrombus Formation Thrombosis initiated by rupture of the plaque, exposing material to subendothelium in the blood ◦ Causes platelet plasma coagulation factor activation which results in fibrin formation. The end result is a thrombus that can obstruct the artery or an embolus breaks off and lodges in the heart or brain, causing tissue death Atherosclerosis in Artery Arterial Thrombus Risk Factors ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Hypercholesterolemia Hypertension Smoking Physical inactivity Obesity Diabetes Inflammatory processes related to atherosclerosis Hyperhomocysteinemia Increased lipoprotein A Oxidized LDL’s Venous Thrombi Red thrombi ◦ Form in veins ◦ Composed of rbc’s trapped in fibrin mesh with few platelets and WBC’s ◦ Form in areas of slow or disturbed blood flow, where venous segments have been exposed to direct trauma Venous Thrombi Occurs when activation of blood coagulation exceeds ability of the anticoagulant/inhibitors and fibrinolytic system to prevent the formation of fibrin. Most occur in veins in lower limbs ◦ Thrombophlebitis= thrombosis of superficial veins of legs ◦ Deep Vein Thrombosis (DVT)=deep veins in limbs and more serious Venous Thromboembolism Venous Thromboembolism (VTE) ◦ Pulmonary embolism (PE) Embolization of lung circulation ◦ Deep vein thrombosis (DVT) Venous Thrombosis Risk Factors ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Venous stasis Vessel wall damage Factor V leiden and protein C resistance Deficiency of AT, Protein C, Protein S, heparin cofactor II Increased PT levels Antiphospholipid antibodies Hyperhomocysteinemia Decreased fibrinolytic activity Malingnancy Surgery Misc( those associated with plaque formation, pregnancy and oral contraceptive use) Thrombophilia Most common causes of death in the United States ◦ Ischemic heart disease ◦ Stroke Inherited or acquired ◦ Conditions that have an increased risk of thrombosis or hypercoagulability Inherited Predisposing genetic defect that results in a tendency for thrombosis Usually associated with venous thrombosis Caused by: ◦ ◦ ◦ ◦ Increased activation of coagulation cascade Defect or decrease in natural inhibitors Abnormalities of fibrinolysis Abnormalities in platelet activation Inherited: Clinical Presentation Venous thromboembolis prior to age 45 Recurrent VTE Family history of VTE Thrombosis in an unusual site ◦ cervical/ visceral veins Inherited States Associated with Thrombosis Antithrombin (AT) deficiency ◦ AT binds thrombin to inhibit coagulation. When deficient thrombin may uncontrollably convert fibrinogen to fibrin clots. ◦ Observe DVT in the leg ◦ Rare, but has severe clinical manifestations Inherited States Associated with Thrombosis Deficiency of Protein C or S ◦ Protein C and S work together to inactivate factors Va and VIIIa ◦ Lack of Protein C or S will result in increased production of thrombin, which generates fibrin ◦ Protein C deficiency=common DVT ◦ Protein S deficiency=risk of ARTERIAL thrombosis Inherited States Associated with Thrombosis Activated Protein C resistance (FVL) ◦ Genetic mutation of factor V (F V Leiden) which causes resistance to the action of Protein C Factor II (Prothrombin) 20210 mutation ◦ Causes increased thrombin generation ◦ Increases risk of venous thrombosis Acquired States Antiphospholipid Antibody Syndrome 1. ◦ ◦ ◦ ◦ Most common acquired thrombophilia Includes the lupus anticoagulant, anticardiolipin antibodies and others are antibodies that prolong phospholipid dependent clotting assays in vitro Antibodies made after certain infections, after exposure to certain medications, and in patients with autoimmune disorders Patients show no bleeding disorder Acquired States 2. Heparin-Induced Thrombocytopenia(HIT) ◦ Autoantibody directed against heparin complexed with platelet factor 4. ◦ Induces platelet activation and aggregation ◦ Patients presents with a thrombocytopenia of < 150 x 109/L 5-14 days after starting heparin therapy Acquired States 3. Thrombotic Microangiopathies (TMA) ◦ Characterized by: Microangiopathic hemolytic anemia Thrombocytopenia Microvascular thrombotic lesions Examples include: DIC, TTP, HUS Activation of platelets without the cascade activating, platelets aggregate in vasculature Acquired States Malignancy ◦ Stasis, activation of blood coagulation and vascular injury play a role ◦ Chemotherapy & surgery increases risk 5. Pregnancy & Oral Contraceptives 6. Postoperative States 7. Hematologic Disorders ◦ MPD: Polycythemia Vera, idiopathic myelofibrosis, essential thrombocythemia 4. Antithrombotic Therapy: 3 Categories Antiplatelet Drugs ◦ Aspirin ◦ Ticlid/Plavix Anticoagulant Drugs ◦ Heparin ◦ Oral Anticoagulants Thrombolytic Drugs ◦ Plasminogen activators are used to lyse thrombi in vivo Streptokinase/Urokinase Antiplatelet Therapy Aspirin ◦ Results in irreversible inhibition of the platelet enzyme cyclooxygenase, which is needed for proper platelet aggregation ◦ This reduces the “stickiness” of platelets ◦ Affects last for the lifetime of the platelets – 7-10 days NSAID drugs (such as ibuprofen) ◦ compete for cycloxygenase and may be used in conjunction with aspirin Antiplatelet Therapy ADP Receptor Antagonist ◦ Suppress platelet aggregation and secretion response to ADP ◦ Examples Ticlopidine- Ticlid Clopidrogrel- Plavix Prasugrel- Effient Therapeutic Anticoagulants Heparin (UFH) and Low Molecular Weight Heparin (LMWH) ◦ Administered IV ◦ Causes immediate inhibition of blood clotting ◦ Accelerates the action of AT to inactivate Thrombin (Ia) Heparin will not work if AT levels are low, thus AT called heparin co-factor ◦ Monitored using the APTT test ◦ Heparin can be neutralized by protamine sulfate ◦ Low molecular weight heparin (LMWH) has less risk and is replacing traditional heparin Therapeutic Anticoagulants Oral anticoagulants ◦ Coumadin (Warfarin, Dicoumarol) ◦ Takes couple days for effects to show ◦ Inhibits production of vitamin K dependent factors (II,VII, IX, X) (Protein C & S) ◦ Monitored using the PT test (since factor VII has the shortest ½ life and becomes deficient first) New Oral Anticoagulant (NOACs) Classified as direct or indirect inhibitors ◦ Direct- bind directly to their target enzyme to block interaction with the substrate Examples- Factor IIa inhibitor Dabigratran “Pradaxa” ◦ Indirect- bind plasma cofactors or accelerate interaction with clotting enzymes Example-Factor Xa inhibitors Rivaroxaban “Xarelto” Apixaban “Eliquis” References McKenzie, Shirlyn B., and J. Lynne. Williams. "Chapter 33." Clinical Laboratory Hematology. 2nd ed. Boston: Pearson, 2010. Print.