Exam II – Some Practice Problems 1. You were asked by your
advertisement

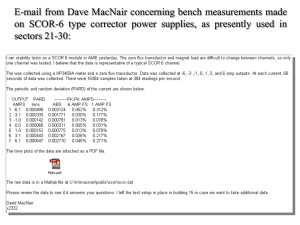
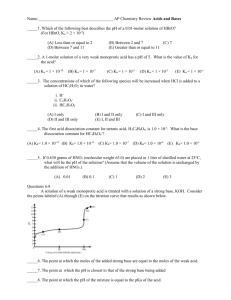
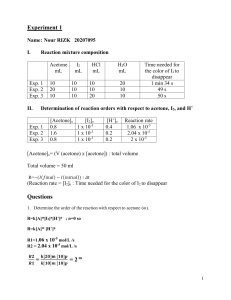
Exam II – Some Practice Problems 1. You were asked by your Biochemistry professor to make a buffer with a pH equal to 3.50. You decide that you will dissolve the conjugate base in 2.00L of 2M weak acid. How many moles of conjugate base do you need to dissolve in the weak acid? Acids to choose from 1. citric acid 2. hypochlorous acid 3. nitrous acid Ka1 = 7.45 x 10-4 Ka = 3.0 x 10-8 Ka = 7.1 x 10-4 2. In lab, your assignment is to titrate 25.00 mL of 0.050 M propanoic acid (Ka = 1.34 x 10-5) with 0.1000 M NaOH using a pH meter. For your pre-lab, you decide that it would be a good idea to predetermine the pH that you will get at the equivalence point. What will the pH be at the equivalence point? 3. You are given an 8.00 ppm formic acid (HCOOH) solution (density of 1.22 g/mL). What is the percent ionization of the HCOOH if the equilibrium molarity of HCOO- is equal to 2.12 x 10-5 M? 4. What pH does the solution need to be to perform a 95% separation of 0.10 M Ce+3 and 0.10 M Ca+2 Ce(OH)3 (s) Ksp = 6.0 x 10-22 Ca(OH)2 (s) Ksp = 6.5 x 10-6 5. Which of the following acidic solutions has the lowest pH? (Circle One) a. 0.0100 M benzoic acid Ka = 6.28 x 10-5 b. 0.100 M butanoic acid Ka = 1.52 x 10-5 c. 0.100 M acetic acid Ka = 1.75 x 10-5 6. Consider the following reaction N2(g) + ½O2(g) N2O(g) K = 2.7 x 10-18 Calculate the numerical value of the equilibrium constant for the reaction below 2N2(g) + O 2(g) 2N2O(g) 7. a. If the Ksp for the reaction below is 1.8 x 10-10, what is the equilibrium concentration of Ag+ for this reaction. SHOW ALL WORK for FULL CREDIT AgCl(s) Ag+(aq) + Cl – (aq) b. What is the equilibrium concentration of Ag+ if you add 0.050M MgCl2? Don’t forget about Thermodynamics!