An example of a lab report
advertisement

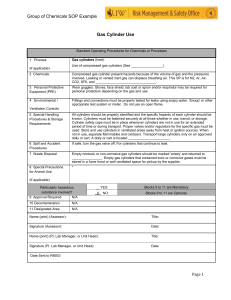
A Detailed Example of a Chemistry Lab Report John Dillinger FOLLOW A real-life example of an academic chemistry lab report Philip Dunay, Honors Chemistry, Mr. Goldstein, 9/14/09 Density Lab Report Introduction: The purpose of this experiment is to find out the density of the four yellow cylinders. To do this we will be getting the density by massing the object and then dividing the mass by its density. It is thought that the density will definitely be over 1.00 because it has to sink in order for us to complete the lab Materials: - Four cylinders - Graduated cylinder - Water (Can be tap water) - Electronic balance - Calculator Procedure: 1. Fill a graduated cylinder with tap water up to 25 ml. 2. Take out your object which you will be testing the density of, in this case the yellow cylinders. 3. Slowly slide in one cylinder and note the amount of ml's the water level changed. 4. To get the difference, subtract 25 from the resulting amount of ml's and record the data. 5. Wash out the test tube and refill it to 25 ml. 6. Repeat steps 1-5 for the remaining three cylinders. 7. Set up your balance in order to mass the four cylinders now. 8. Zero the balance in order to get rid of any error and then set the object lightly on the balance and wait for it to stabilize. When its not moving anymore, record the mass of the cylinder in grams. 9. Repeat step 8 for the remaining three cylinders. 10. When you have the four masses and four volumes, it is time to find the density. To find the density, divide mass by volume and round to the nearest number of significant digits. 11. Repeat step 10 for the remaining three cylinders. You now have the density for all four cylinders and now it is time to graph the data and report the results. Conclusion: The purpose of the experiment was to find out the density of the four given cylinders. To do this the mass was found and the divided by the volume for all four cylinders, resulting in the density. The end result was 1.22 for the g/ml and the known answer was 1.23 resulting in the amount of 0.81% error. Having the density of 1.23, this proved the hypothesis correct as it was estimated only to be more than 1.00. A possible source of error may have been slightly incorrect estimations when it comes to significant figures. An improvement to this lab would be to have multiple results for one cylinder, making it so they could be averaged into a possible more accurate result.