pola27655-sup-0001-suppinfo
advertisement

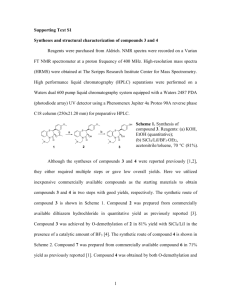
Supporting Information Facile Fabrication of Fast Recyclable and Multiple Self-healing Epoxy Materials through Diels-Alder Adduct Cross-linker Xiao Kuang1, Guoming Liu1, Xia Dong1, Xianggui Liu1, Jianjun Xu2, Dujin Wang1* 1 Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China 2 DSM Resolve, P. O. Box 18, 6160 MD Geleen, the Netherlands Synthesis of Tert-butyl (2-(2, 5 - dioxo-2, 5-dihydro-1H-pyrrol - 1-yl)ethyl) Carbamate (1). N-Boc-ethylenediamine (16.01 g, 100 mmol) in CH2Cl2(100 mL) was added under stirring to maleic anhydride (10.49 g, 107.1 mmol) in CH2Cl2/acetone (100 mL/5mL) at 0 °C and stirred for 3h at room temperature. After the reaction, CH2Cl2 was evaporated and the residue was dissolved in acetone (250 mL) and acetic anhydride (12.2 mL, 130 mmol), then nickel (II) acetate tetrahydrate (0.450 g, 1.8 mmol) and triethylamine (2.7 mL, 19.4 mmol) were added to the solution under stirring. The mixture was heated at 60 °C for 24h and then cooled to room temperature. Acetone was evaporated and the residue was poured into ice water (500 mL) and stirred for 30 minutes. The mixture was filtered and washed with water (2×30 mL). After drying, Compound 1 was yielded (20.41 g, 85%) as white powder. 1HNMR [400MHz, CDCl3, δ(ppm)]: 6.71 (s, 1H), 4.73 (s, 1H), 3.70 – 3.59 (m, 2H), 3.33 (dd, J = 10.8, 5.5 Hz, 2H), 1.42 (d, J = 16.8 Hz, 9H). 13C NMR [300MHz, CDCl3, δ(ppm)]: 171.19, 156.34, 134.59, 79.94, 39.84, 38.42, 28.74. ATR (KBr/cm-1): 3109, 3088, 2982, 1703, 1681, 1434, 1368, 1168, 845, 692. ESI-MS (m/z): [M+Na]+ calcd. C11H16N2O4Na, 263. 1; found 263.1. 1 Figure S1. 1H NMR spectrum of Compound 1. Synthesis of Tert-butyl (furan-2-ylmethyl) Carbamate (2). The Compound 2 was synthesized according to reference[1]. Furfurylamine (10.60 g, 0.109 mol) in CH2Cl2 (90 mL) was added under stirring to di-tert-butyl dicarbonate (25.20 g, 0.115 mol) in CH2Cl2 (90 mL) at 0 °C. The solution was stirred at room temperature for 1 day. After the reaction, CH2Cl2 was evaporated and EtOAc (50mL) was added to the solution. The solution was washed with saturated Na2CO3 solution (2 × 40 mL) and dried over anhydrous Na2SO4. The solution was concentrated to yield Compound 2 (21.55 g, 99 %) as yellow crystal. 1HNMR [400MHz, CDCl3, δ(ppm)]: 7.34 (d, J = 1.1 Hz, 1H), 6.34 – 6.27 (m, 1H), 6.20 (d, J = 2.5 Hz, 1H), 4.84 (s, 1H), 4.30 (d, J = 5.3 Hz, 2H), 1.45 (s, 9H). C NMR [300MHz, CDCl3, δ(ppm)]: 155.90, 152.41, 142.26, 110.60, 107.15, 79.92, 77.38, 38.02, 13 28.65. ATR (KBr/cm-1): 3350, 2981, 2935, 1717, 1508, 1395, 1371, 1249, 1213, 1171, 737. ESI-MS (m/z): [M+Na]+ calcd. C10H15NO3Na, 220.1; found 220.1. 2 Figure S2. 1H NMR spectrum of Compound 2. Synthesis of Tert-butyl(2-(2-aminoethyl)-4-(aminomethyl)-3a, 4, 7, 7a – tetrahydro-1H-4, 7 – epoxyisoindole-1, 3(2H)-ethyl) Carbamate (3). The Compounds 1 (21.50 g, 109.3 mmol) and 2 (21.71 g, 90.4 mmol) were added in EtOAc (100 mL). The solution was heated at 80 °C for 14h and another 10h at 70 °C. After the solution was cooled, the upper solution layer was separated and the residual solid was washed with cold diethyl ether (3 × 60 ml) to yield Compound 3 (33.56 g, 85 %) as white solid. The compound 3 is exo isomer. 1HNMR [400MHz, CDCl3, δ (ppm)]: 6.52 (dd, J = 21.3, 5.4 Hz, 2H), 5.25 (s, 1H), 5.20 (d, J = 1.4 Hz, 1H), 4.71 (s, 1H), 3.83 (dd, J = 14.6, 6.4 Hz, 1H), 3.71 (ddd, J = 12.6, 7.8, 4.6 Hz, 2H), 3.55 (dt, J = 13.5, 4.9 Hz, 2H), 3.30 (d, J = 8.4 Hz, 2H), 2.97 (d, J = 6.4 Hz, 1H), 2.89 (d, J = 6.4 Hz, 1H), 1.43 (d, J = 15.4 Hz, 18H). 13C NMR [300MHz, CDCl3, δ (ppm)]: 176.33, 175.6, 156.3, 156.1, 139.0, 137.2, 91.32, 81.14, 79.96, 50.45, 48.46, 40.04, 39.00, 38.62, 28.64. ATR (KBr/cm-1): 3372, 2978, 2935, 1772, 1703, 1527, 1440, 1395, 1367, 1336, 1250, 1171, 692, 864. ESI-MS (m/z): [M+Na]+ calcd. C21H31N3O7Na, 460.2; found 460.2. 3 Figure S3. 1H NMR spectrum of Compound 3. 4 Figure S4. DSC heating thermograms of Compound 3: (a) as synthesized, (b) after heating at 170 °C for 2 min and annealing at 75 °C for 2 h then cooling to room temperature, and (c) after heating at 170 °C for 2 min and quenching to room temperature. The heating rate was 10 °C min-1. Inserted was the magnified curve (c) in temperature range of 100 -160 °C. Figure S5. DSC heating curves of unreacted novolac epoxy oligomer (F-44, Shanghai resin factory co. LD) and diamine DA adduct cross-linker mixture (a) and as-cured novolac epoxy polymer (b). 5 Figure S6. Images of solubility tests: (a) as-cured epoxy polymer swelling in DMF at room temperature (left) and full dissolving in DMF at 130 °C after 20 min (right), (b) 1st recycled epoxy polymer swelling in DMF at room temperature (left) and full dissolving in DMF at 150 °C after 30min (right). 6 Figure S7. Images for color change: (a) cured mixture of DGEBA and furfurylamine (NH: epoxide = 0.96 : 1, the same below) at 60 °C for 15 h (light yellow viscous liquid); (b) cured (a) for additional 120 °C for 2h (light yellow brittle solid); and (c) cured mixture of (a) and bismaleimide (furan/maleimide =1/1) (first dissolving and mixing in CHCl3 and then evaporating the solvent) at 120 °C for 1 h (dark red brittle solid). Figure S8. TGA results of as-cured and 1st recycled epoxy polymers. REFERENCES 1. N. Bai, K. Saito, G. P. Simon, Polym. Chem. 2013, 4, 724-730. 7