Electron Configuration
advertisement

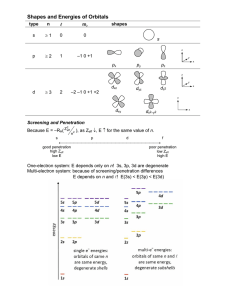
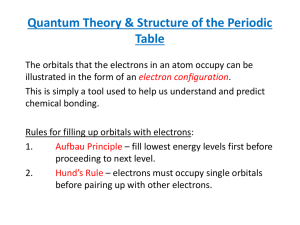
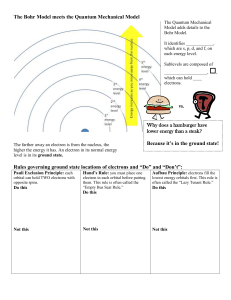
Electron Configuration Methods for expressing electron locations: 1. Configuration 2. Orbital Notation ELECTRON CONFIGURATION • Electrons find homes in orbitals • Electrons fill orbitals from lowest energy level FIRST, to highest energy level, LAST. • REMEMBER! – – – – s holds 2 – color the s blockORANGE p holds 6 – color the p blockPURPLE d holds 10 – color the d block GREEN f holds 14 – color the f block BLUE GRAB COLORS! LET’S PRACTICE • • • • Let’s write the electron configuration for Mg. What is the atomic number of Mg? _____ 12 How many electrons does Mg have? ______ 12 Write the electron configuration for Mg. 1s22s22p63s2 RULES 1. e- occupy orbitals of lowest energy first 2. orbitals hold up to two electrons of opposite spin (clockwise – counterclockwise) 3. one e- enters each orbital until all orbitals have 1 e- with the same spin direction, THEN it’s paired with e- with different spin (LONERS FIRST PARTNERS SECOND) ORBITAL NOTATION We can also use diagrams with arrows to show electrons and their spin in a diagram called orbital notation. Each block represents an orbital (like an electron parking spot). 1 atom of Li # of P+ = 3 # of e- = 3 1 atom of F # of P+ = 9 # of e- = 9 OCCUPY LOWEST ENERGY FIRST BEFORE MOVING TO HIGHER ENERGY LEVEL OPPOSITE SPIN LONERS FIRST PARTNERS SECOND ORBITAL NOTATION YOU TRY SULFUR!



![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)