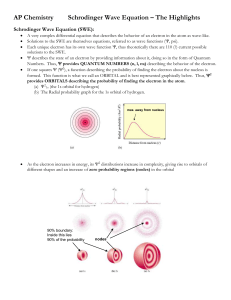
6) cobalt [Ar] 4s 2 3d 7
advertisement
![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-768x994.png)
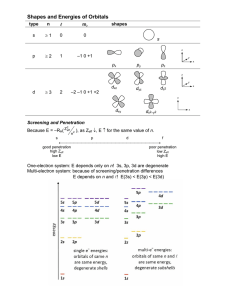
Name: ____________________ Period: ____ Date: ________________ Electron Configuration Practice Worksheet Write the electron configurations of the following elements. For the first two, also include the orbital notation. 1s22s22p63s1 1) sodium 2) iron 3) bromine 1s22s22p63s23p64s23d104p65s1 4) barium 5) neptunium 1s22s22p63s23p64s23d6 1s22s22p63s23p64s23d104p5 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s75f5 Write the noble gas configurations of the following elements: 6) cobalt [Ar] 4s23d7 7) silver [Kr] 5s24d9 8) tellurium 9) radium 10) lawrencium [Kr] 5s24d105p4 [Rn] 6s2 [Rn] 6s24f145d1 Determine what elements are denoted by the following electron configurations: 11) 1s22s22p63s23p4 sulfur 12) 1s22s22p63s23p64s23d104p65s1 rubidium 13) [Kr] 5s24d105p3 antimony 14) [Xe] 6s24f145d6 osmium 15) [Rn] 7s25f11 nobelium Fill in the table below using all that we have learned in class: Element Electron Configuration Noble Gas # Lewis Dot Configuration Valence structure Electrons K 1s22s22p63s23p64s1 Y 1s22s22p63s23p64s23d104p65s24d1 [Kr] 5s24d1 2 1s22s22p63s23p3 [Ne] 3s23p3 5 1s22s22p2 [He] 2s22p2 4 P C [Ar] 4s1 1 Explain the Aufbau Principle. Orbitals of lower energy get filled before those of higher energy. 1s is filled before 2s. Which orbitals are filled in immediately after the 3d? 4p Which orbital is filled in immediately after the 5s? 4d Define Hund’s Rule. Each orbital of equal energy gets one electron before doubling up. Of the following pairs of orbitals, which is higher energy? 3d and 4s 5d and 5f