
Structure and Function of the Renal Tubule (Physiology)
Structure and function of the renal tubule - Dr Daniel Berwick Session Summary This sessions describes what happens to the fluid filtered by the glomerulus as it passes through the tubule and collecting duct. Put simply, you will learn about the processes by which a liquid that is very similar to plasma (bar the cellular content and much of the protein) is converted into wee. The content in the session was adapted from a previous lecture by Dr Suman Rice. Learning outcomes At the end of this session you will be able to understand: Mechanisms by which glomerular filtrate is altered to produce urine Reabsorption from and secretion into the glomerular filtrate within the tubule Active and passive transport in the renal tubule Techniques to investigate renal active and passive transport The structural components of tubule & the function of each segment Counter-current exchange and the Loop of Henle Abnormalities of urine and kidneys Session resources Structure and Function of the Renal Tubule 2023-2024.pptxDownload Structure and Function of the Renal Tubule 2023-2024.pptx Session activities Parts 1-6 of this session are covered within the PowerPoint slides and lecture / recording. A seventh part - a table describing common kidney pathologies - is included on this Canvas page. Part 7 - Diseases and pathologies of the kidney The table below describes some common disease of the kidney Kidney Pathologies Polycystic Kidney Disease (PKD) Polycystic kidney disease is a genetic disorder characterised by growth of numerous cysts (see image) PKD.png Glomerulonephritis (GN) Inflammation of glomeruli of some or all of million nephrons in kidney Can be primary or secondary to systemic disease like diabetes mellitus This is a distinct condition to nephrotic syndrome (you will learn more about this in the next lecture) Glomerulonephritis.png Diseases of the tubule Obstruction (reducing glomerular filtration) Impairment of transport functions (reducing water & solute reabsorption) eg. Fanconi’s syndrome Acquired Kidney Diseases Hypertension Kidneys regulate ECF volume and hence influence blood pressure Compensatory mechanisms in response to high BP can lead to chronic kidney damage Congestive Cardiac Failure Fall in cardiac output ⇒ renal hypoperfusion ⇒ registered as hypovolaemia, compensation results in pulmonary oedema Diabetic nephropathy As a consequence of diabetes (poor glucose control and hypertension), filtering system of kidneys gets destroyed over time Lithium treatment results in acquired nephrogenic diabetes insipidus* Due to reduction of AQP2 expression * Diabetes insipidus (DI) is a condition characterized by excessive thirst and excretion of large amounts of severely diluted urine, with reduction of fluid intake having no effect on the concentration of the urine. There are different types of DI, each with a different set of causes. The most common type in humans is the neurological form, called Central DI (CDI), which involves a deficiency of arginine vasopressin (AVP), also known as antidiuretic hormone (ADH). The second common type of DI is nephrogenic diabetes insipidus (NDI), which is due to kidney or nephron dysfunction caused by an insensitivity of the kidneys or nephrons to ADH. Additional resources Essays on Classic Papers: “Micropuncture: unlocking the secrets of renal function” J.M. Sands (2004) Am.J.Physiol Renal Physiol 287:F866-867 “Experimental Validation of the countercurrent model of urinary concentration” JA Schafer (2004) Am.J.Physiol Renal Physiol 287:F861-F863 General Physiology Textbooks “Textbook of Medical Physiology” Guyton & Hall (11th edition) Elsevier Saunders (2006) “Renal Function” H. Valtin & J Schafer (3rd edition) Little, Brown & Co. (1995) “Vander's Renal Physiology” DC Eaton & JP Pooler (6th edition) Lange Physiology (2006) http://www.colorado.edu/intphys/Class/IPHY3430-200/countercurrent_ct.htmlLinks to an external site. http://www.edren.org/pages/edreninfo.phpLinks to an external site. Why urea is reabsorbed Session conclusion Hopefully you can now see the tubule and collecting duct less as of a series of pipes that eventually plumb into your bladder, and more as part of a factory. They take in a filtered fluid that is similar to plasma and begin modifying it, principally by two processes: reabsorption and secretion. Reabsorption is vital for humans (and any species with a kidney) to retain key nutrients and water, while secretion provides another way to get rid of unwanted molecules from the blood (i.e. it does not all happen at the renal corpuscle!). By the end of the process, the fluid has been converted into urine. But not only that, the collecting duct is also able to control how concentrated (or dilute) that urine is, by governing water reabsorption. The image below summarises the tubule and duct of a nephron as a factory. Notice how the image also represents the gradient of osmomolarity (or osmomolality!) within the medulla and how this facilitates water retention via osmosis.
-
What are the learning outcomes for understanding the renal tubule structure and function? (7)
𖹭 Mechanisms by which glomerular filtrate is altered to produce urine
𖹭 Reabsorption from and secretion into the glomerular filtrate within the tubule
𖹭 Active and passive transport in the renal tubule
𖹭 Techniques to investigate renal active and passive transport
𖹭 The structure of the tubule and the function of each segment
𖹭 Counter-current exchange and the Loop of Henle
𖹭 Abnormalities of urine and kidneys
-
What is glomerular filtrate (GF), also known as ultrafiltrate? (2)
𖹭 Plasma ≈ GF except it contains no cells and very little protein
𖹭 Plasma ≠ urine
-
Why and how does plasma differ from urine? (1)
𖹭 The selective modification of glomerular filtrate as it passes through the tubule
-
What clue indicates the selective modification process of glomerular filtrate? (2)
𖹭 GF is formed at 120ml/min
𖹭 Urine flow is approximately 1ml/min
-
What is the reason for the significant reduction in flow rate from glomerular filtrate to urine? (1)
The answer lies in the selective modification of glomerular filtrate as it passes through the renal tubule.
-
Picture demonstrating modifications of the ultrafiltrate:
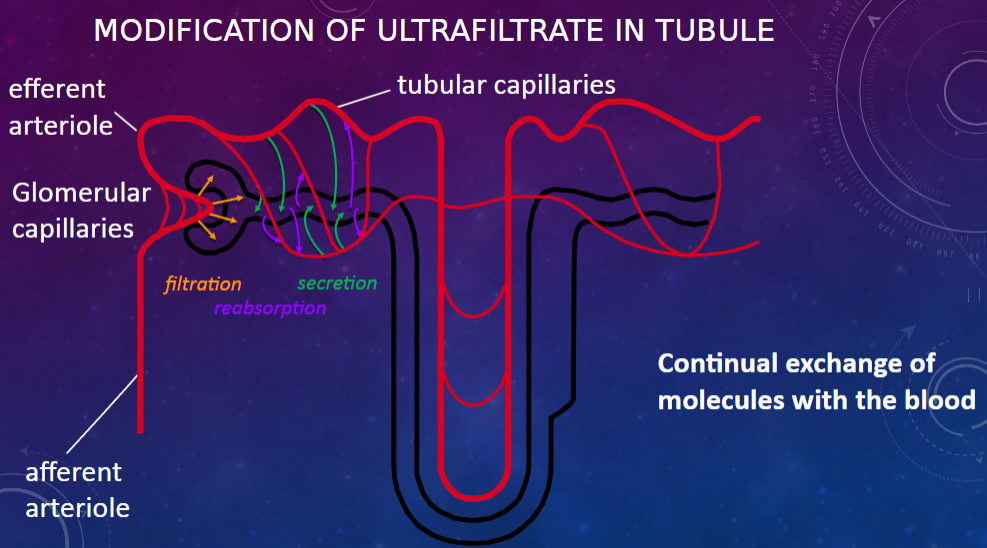
-
Picture demonstrating reabsorption and secretion:
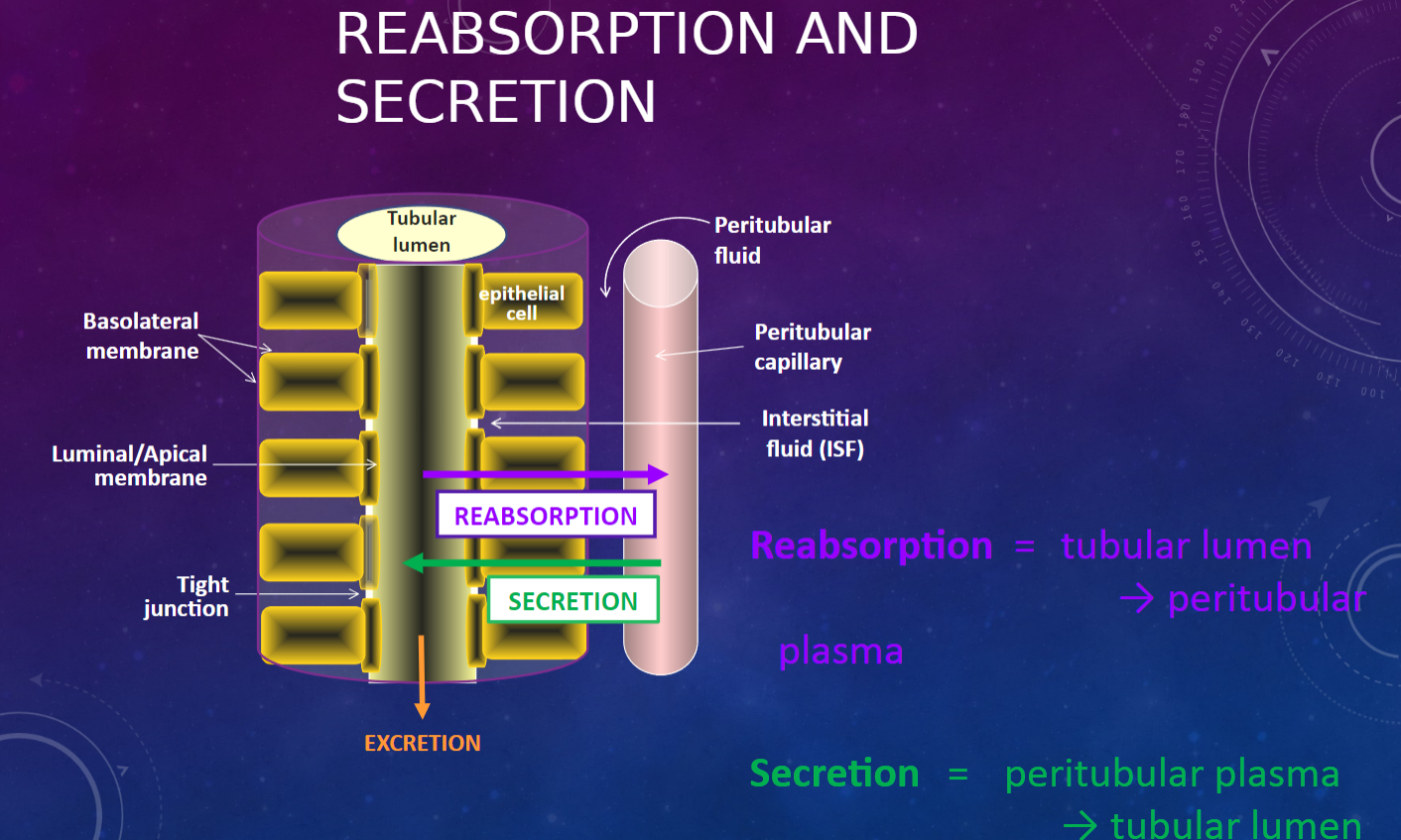
-
What is active transfer or primary active transport? (3)
𖹭 Moving molecule/ion against concentration gradient (low → high)
𖹭 Operates against electrochemical gradient
𖹭 Requires energy - driven by ATP
-
What is passive transfer (or flux)? (2)
𖹭 Passive movement down concentration gradient (requires suitable route)
𖹭 Removal of one component concentrates the other components
-
What is co-transport or secondary active transport? (4)
𖹭 Movement of one substance down its concentration gradient generates energy
𖹭 Allows transport of another substance against its concentration gradient
𖹭 Requires “carrier” protein
𖹭 2 types: symport and antiport
-
What is a symport? (2)
𖹭 Two molecules move in the same direction
𖹭 Example: Na+-glucose
-
What is an antiport? (2)
𖹭 Two (or more) molecules move in opposite directions
𖹭 Example: Na+-H+
-
What mechanisms are involved in transcellular transport in the renal tubule? (2)
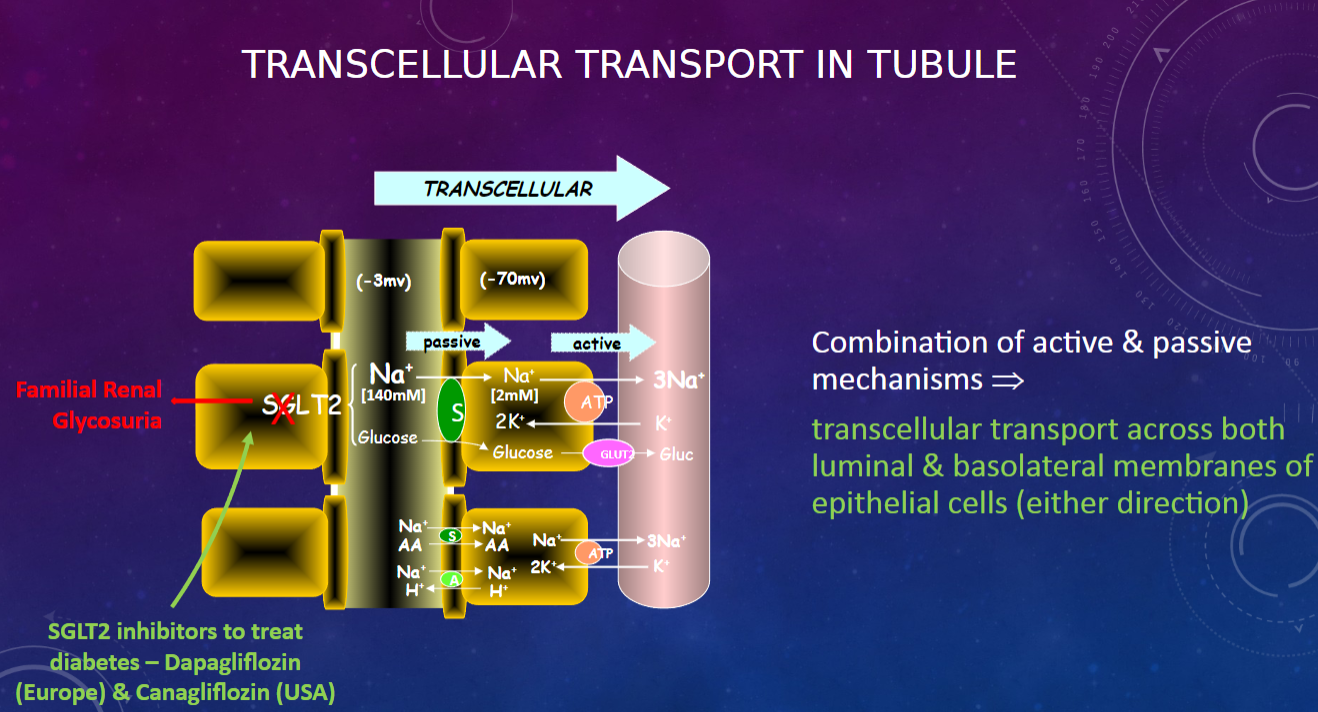
𖹭 Combination of active and passive mechanisms
𖹭 Transcellular transport occurs across both luminal and basolateral membranes of epithelial cells (in either direction)
-
Why is the Na+-K+ ATPase pump important? (3)
𖹭 It is required to maintain high intracellular potassium and low intracellular sodium.
𖹭 This function is essential in all cell types.
𖹭 It is one of the body's biggest uses of ATP.
-
What are the techniques used to investigate tubular function? (3)
𖹭 Clearance studies
𖹭 Micropuncture & Isolated Perfused Tubule
𖹭 Electrophysiological Analysis
-
How are these techniques applied? (1)
𖹭 Clearance studies are applied to humans and are primarily observational.
𖹭 Micropuncture & Isolated Perfused Tubule, as well as Electrophysiological Analysis, are applied to laboratory animals and are mechanistic in nature.
-
What is micropuncture?
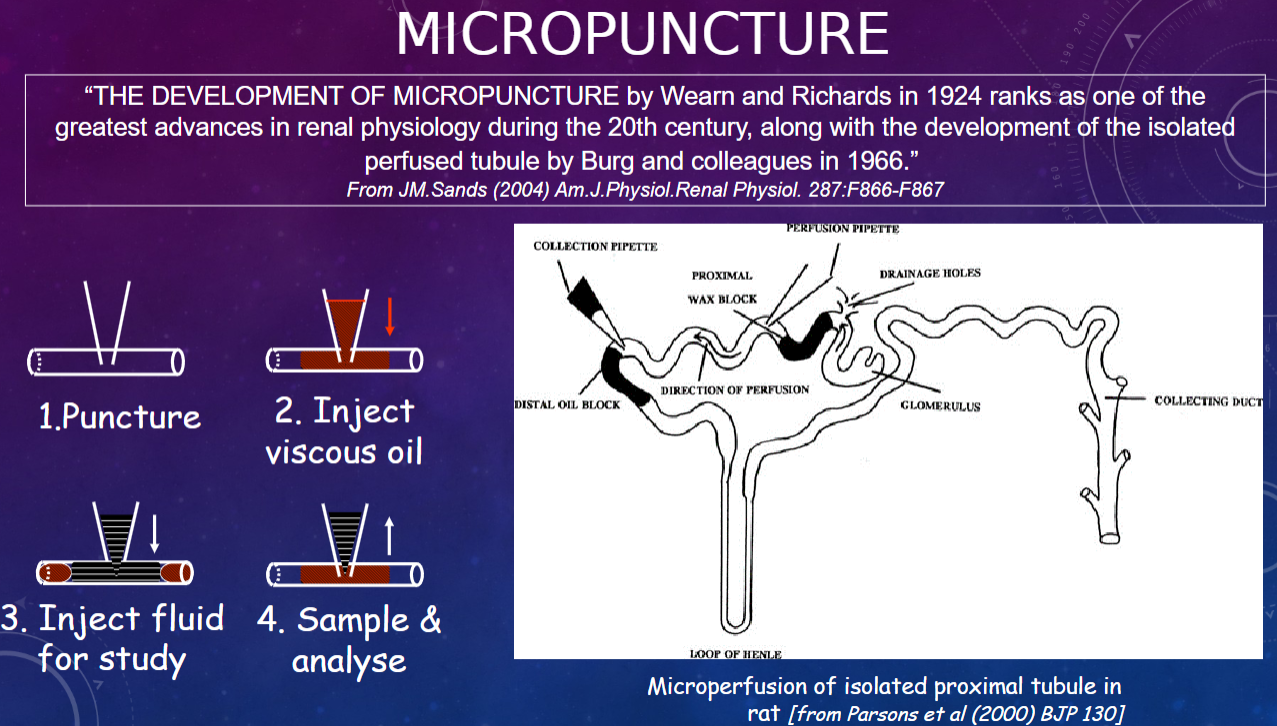
𖹭 Micropuncture is a technique developed by Wearn and Richards in 1924, which is considered one of the greatest advances in renal physiology during the 20th century
-
What is the significance of micropuncture in renal physiology?
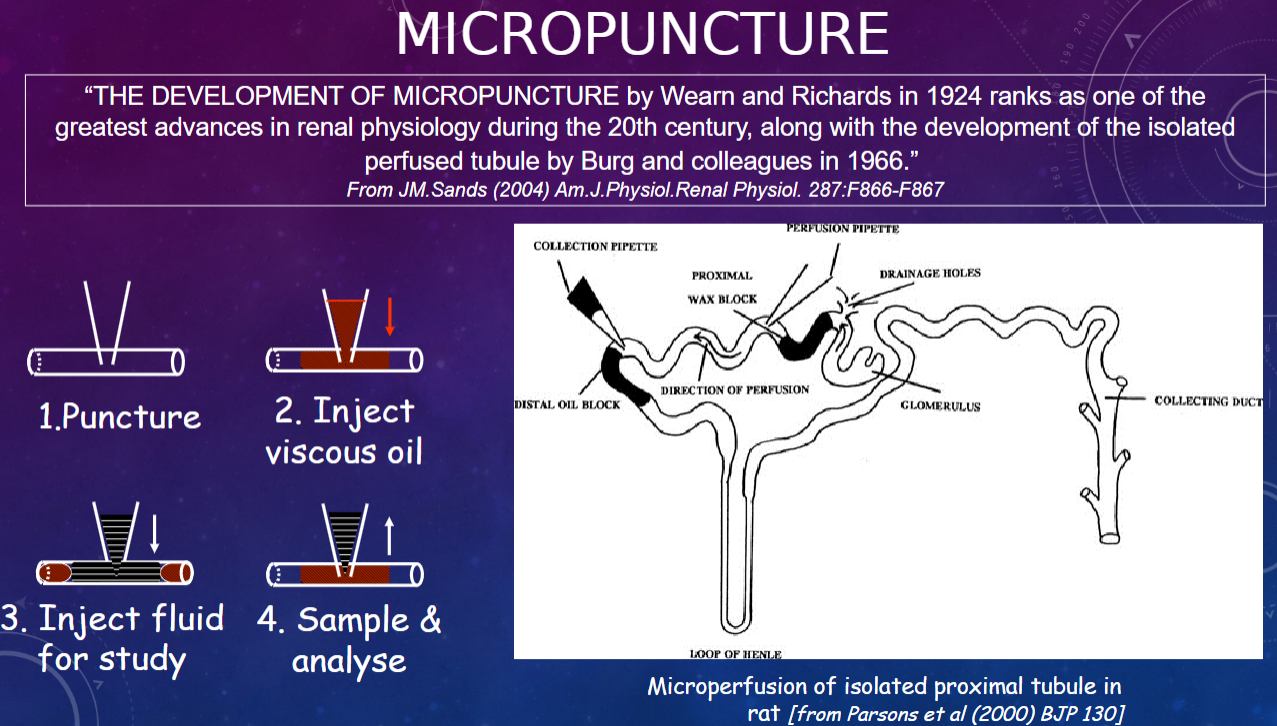
𖹭 Micropuncture allows for precise puncturing of individual nephrons to collect samples directly from specific segments of the nephron, enabling detailed study of renal function at the microscale.
-
How is electrical potential utilized in electrophysiology studies?

𖹭 Electrical potential is combined with microperfusion to alter the potential difference (PD).
-
What is measured in electrophysiological studies related to potential difference (PD)?

𖹭 Electrophysiological studies measure whether ions are moving with or against the electrochemical gradient.
-
What aspect of ion movement is investigated in electrophysiological studies?

𖹭 The studies aim to determine if ion movement is actively transported.
-
What is patch clamping in electrophysiology?
𖹭 Patch clamping is a technique where current flow through individual ion channels is measured.
-
How is electrical resistance measured in patch clamping?
𖹭 Electrical resistance is measured across a patch of cell membrane, which changes when channels open or close.
-
What does patch clamping allow researchers to study?
𖹭 Patch clamping enables the investigation of different types of ion channels and their response to drugs and hormones.
-
Picture demonstrating the 2 types of nephron:
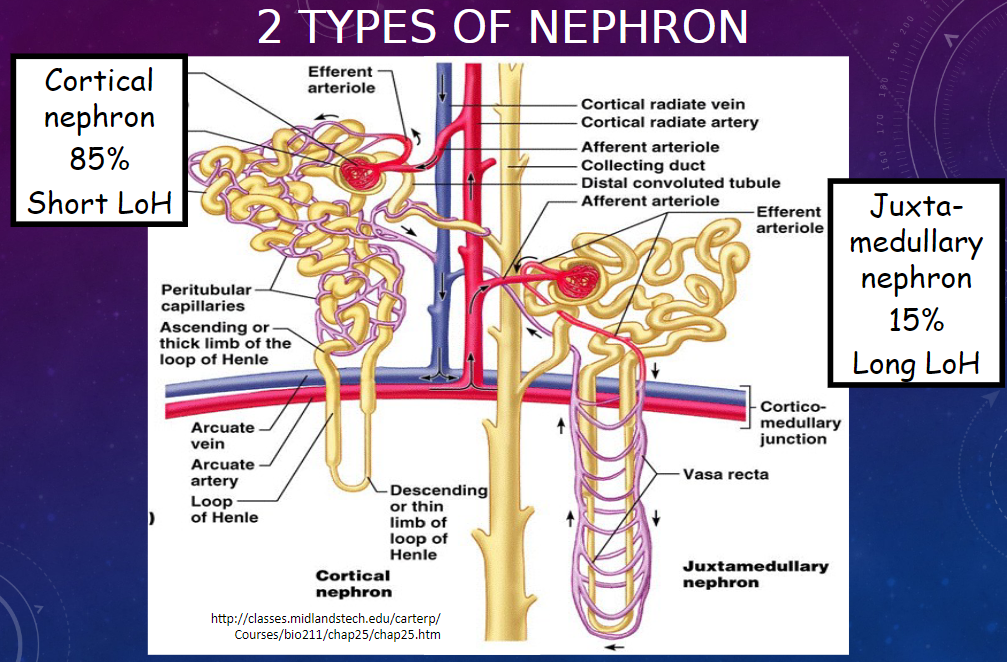
-
What are some unique characteristics of the kangaroo rat's renal system?

𖹭 Barely drinks!
𖹭 Produces the most concentrated urine of any mammal.
𖹭 Recovers almost all dietary water due to remarkably long loops of Henle.
-
What are the main characteristics of the proximal convoluted tubule (PCT)? (2)
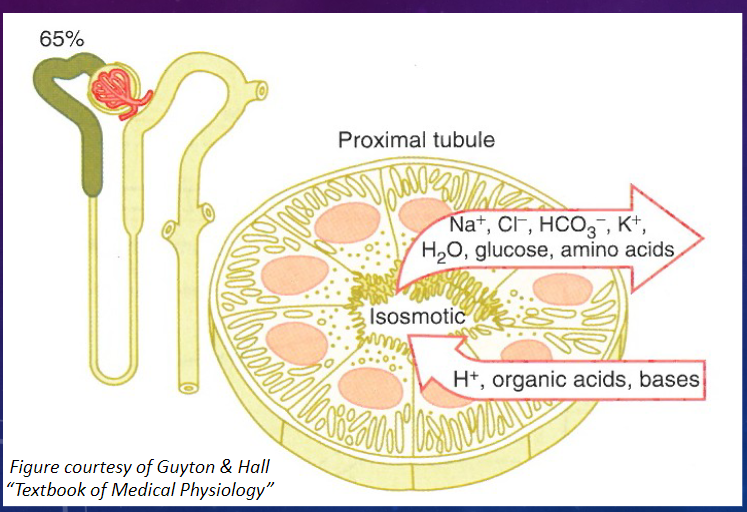
𖹭 The PCT is directly adjacent to Bowman’s capsule.
𖹭 It has a high capacity for reabsorption.
-
What are the characteristics of the epithelial cells in the PCT? (2)
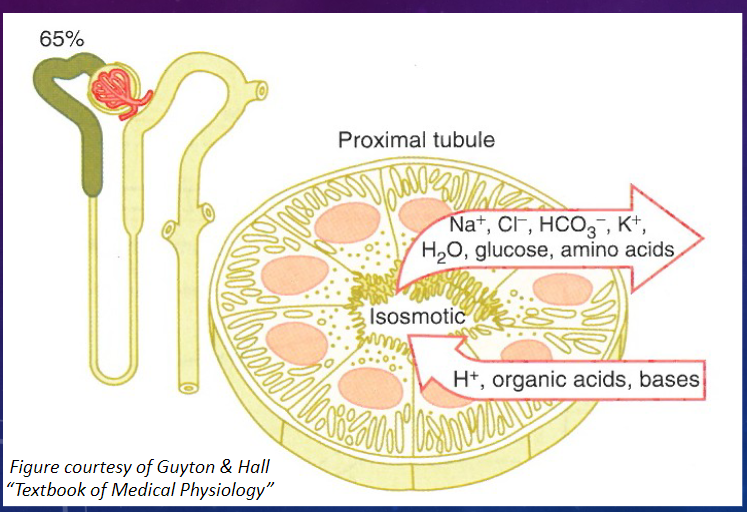
𖹭 Epithelial cells are highly metabolic, containing numerous mitochondria for active transport.
𖹭 There is an extensive brush border on the luminal side, providing a large surface area for rapid exchange.
-
What is the major function of the proximal convoluted tubule (PCT)? (2)
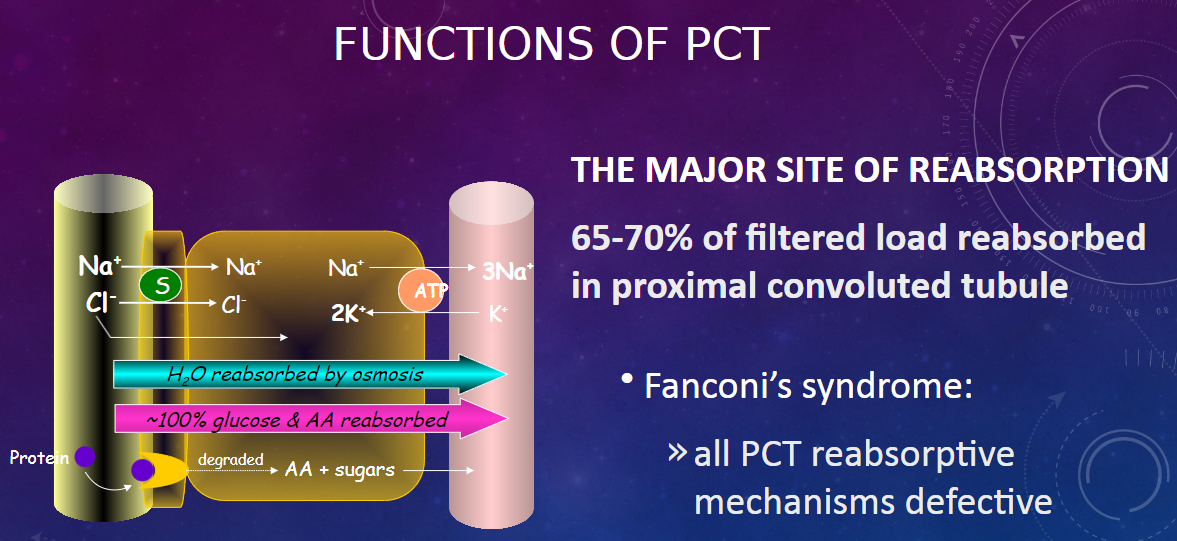
𖹭 The PCT is the major site of reabsorption in the nephron.
𖹭 Approximately 65-70% of the filtered load is reabsorbed in the proximal convoluted tubule.
-
What is Fanconi’s syndrome, and how does it relate to the PCT? (2)
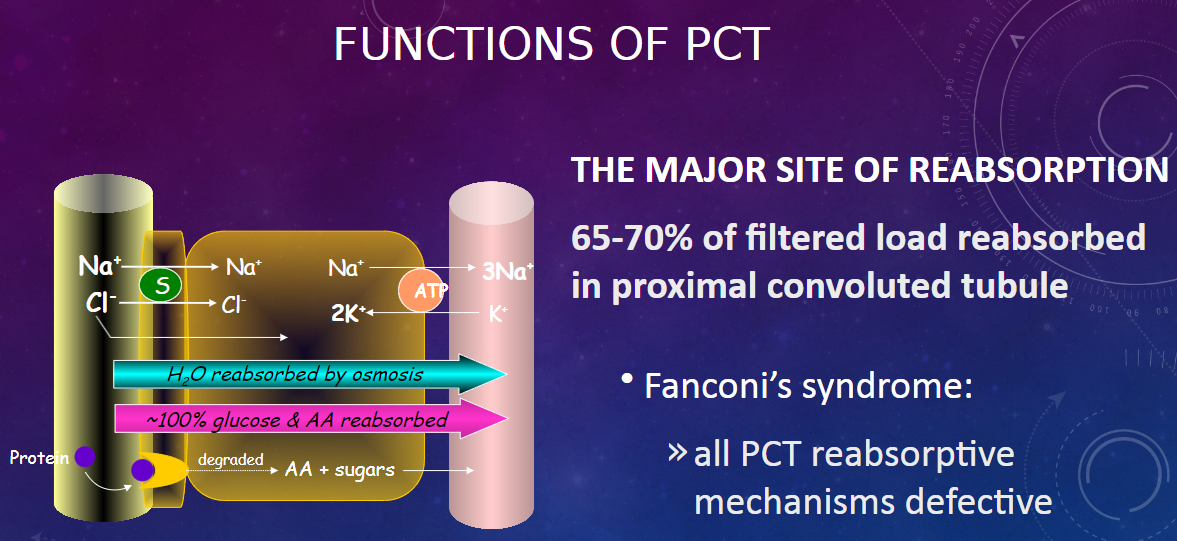
𖹭 Fanconi’s syndrome is characterized by defective reabsorptive mechanisms in all PCT.
𖹭 This condition affects the normal functioning of the proximal convoluted tubule, leading to impaired reabsorption processes.
-
What are the three distinct segments of the Loop of Henle? (1)
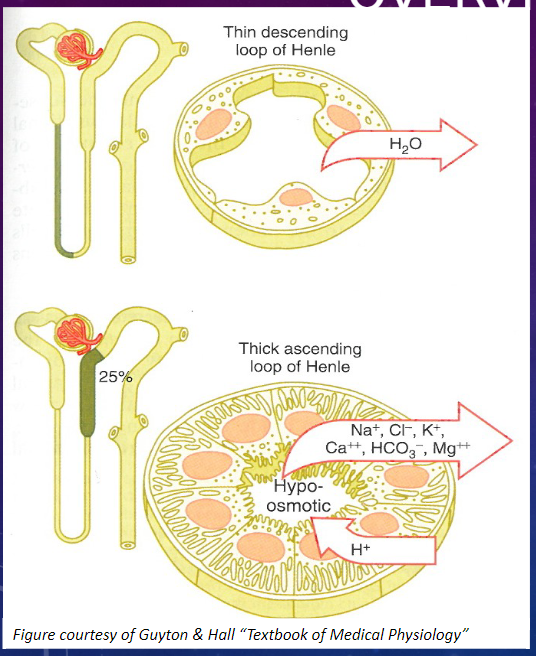
𖹭 The Loop of Henle consists of three segments:
Thin Descending
Thin Ascending
Thick Ascending
-
What are the characteristics of the epithelial cells in the thin segments of the Loop of Henle? (2)
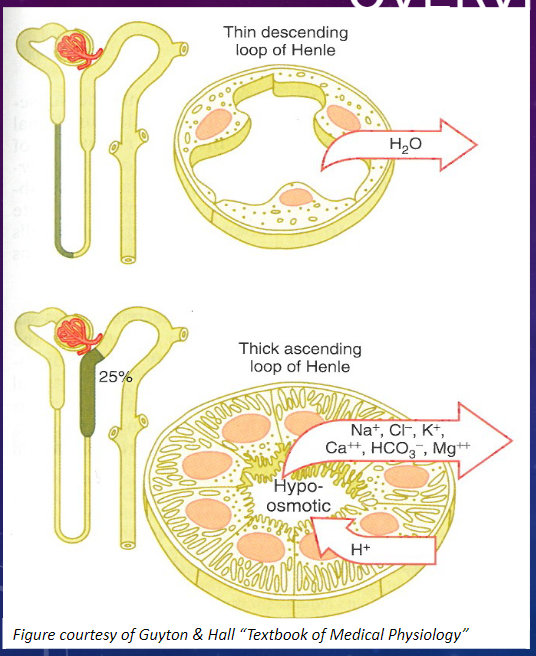
𖹭 Thin epithelial cells with no brush border and few mitochondria.
𖹭 They exhibit low metabolic activity.
-
What are the characteristics of the epithelial cells in the thick segment of the Loop of Henle? (3)
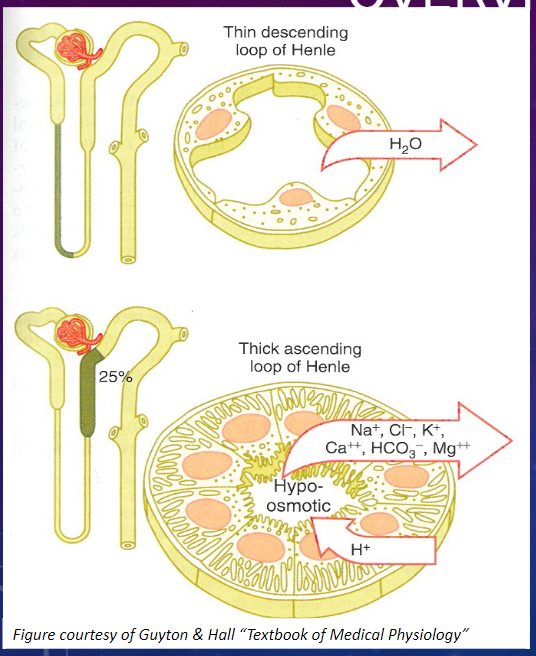
𖹭 Thick epithelial cells with extensive lateral intercellular folding, few microvilli, and many mitochondria.
𖹭 They exhibit high metabolic activity.
-
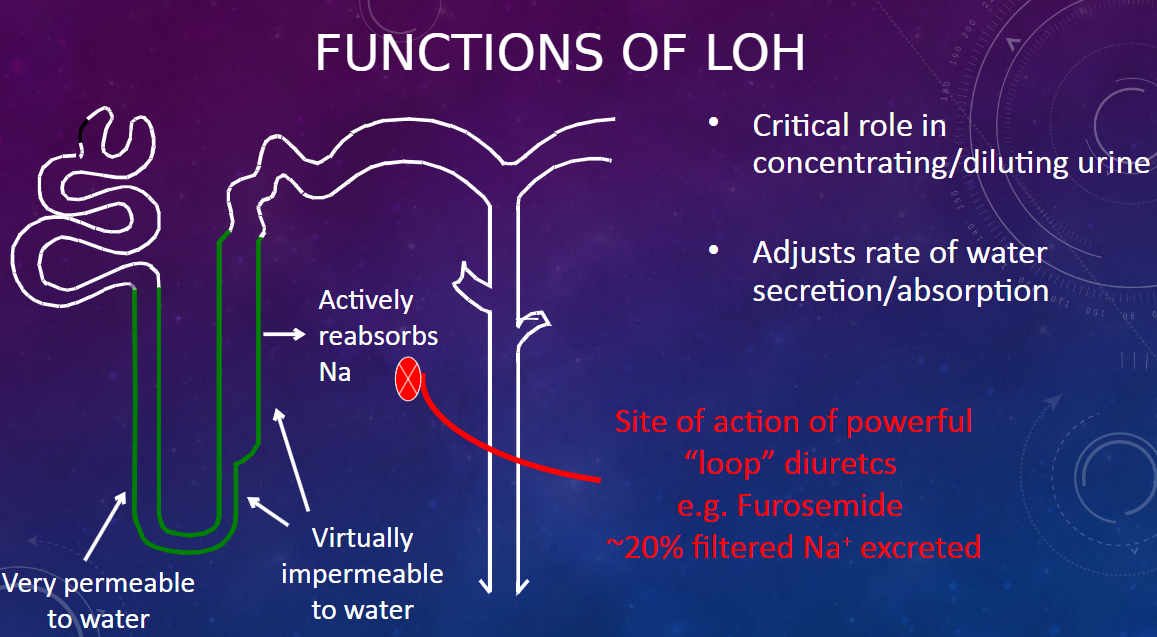
Picture demonstrating the functions of the LOH:
-
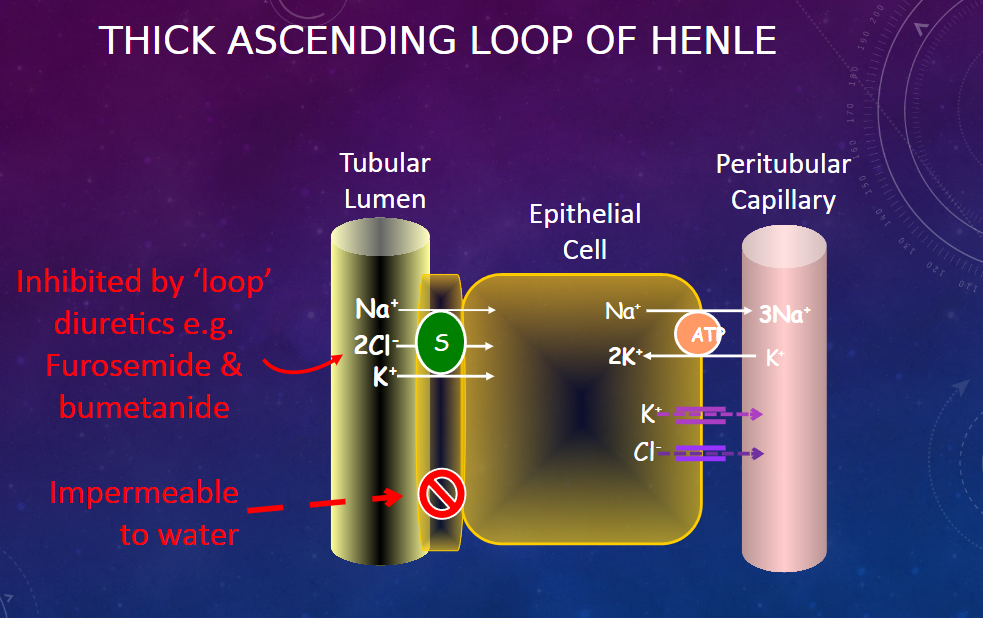
Picture demonstrating the thick ascending LOH:
-
How does the Loop of Henle (LoH) contribute to the medullary osmotic gradient? (1)
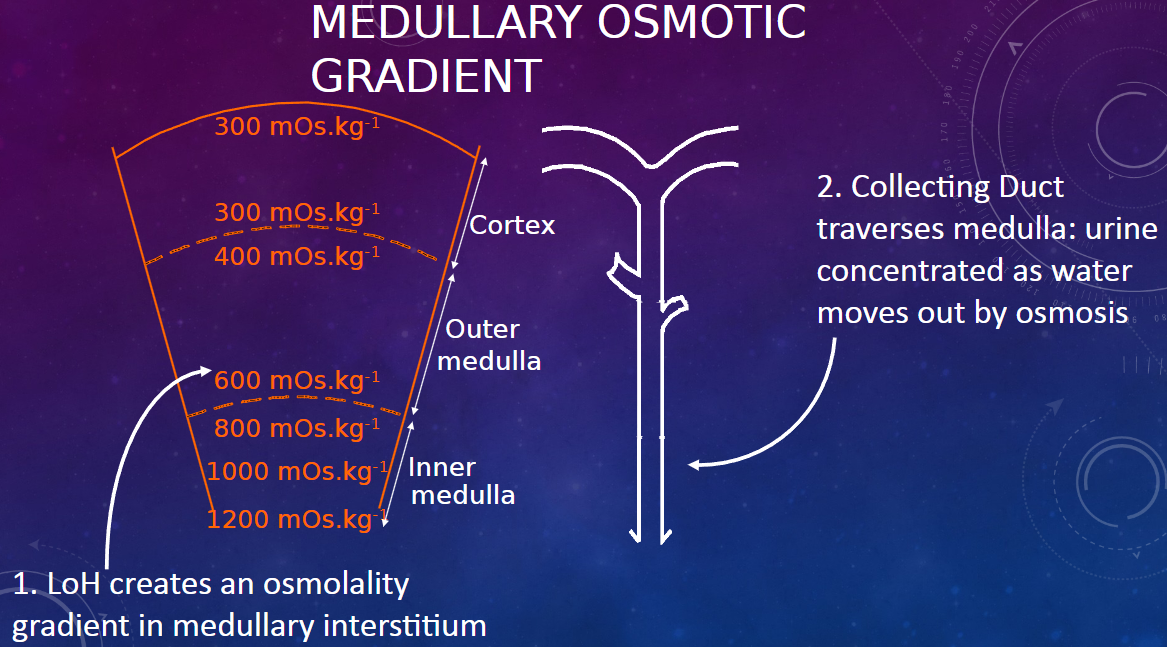
𖹭 The Loop of Henle creates an osmolality gradient in the medullary interstitium.
-
How does the collecting duct contribute to urine concentration in the medulla? (1)
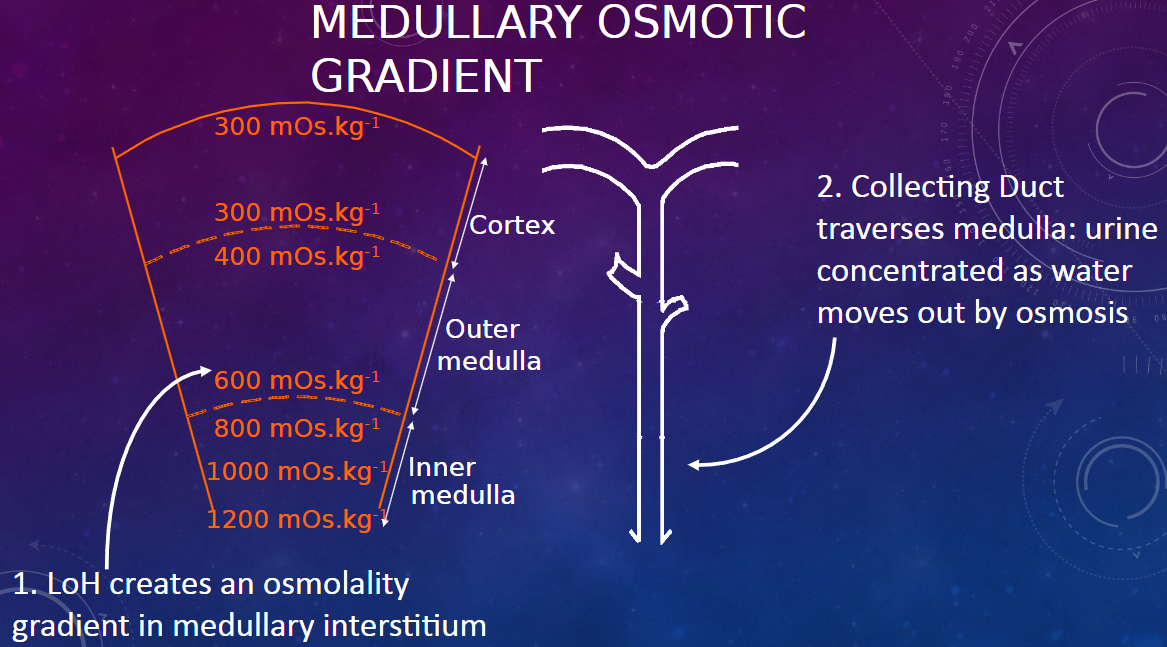
𖹭 As the collecting duct traverses the medulla, urine becomes concentrated as water moves out by osmosis, facilitated by the osmotic gradient created by the Loop of Henle.
-
What is the permeability of the vasa recta (VR) to solutes and water? (1)
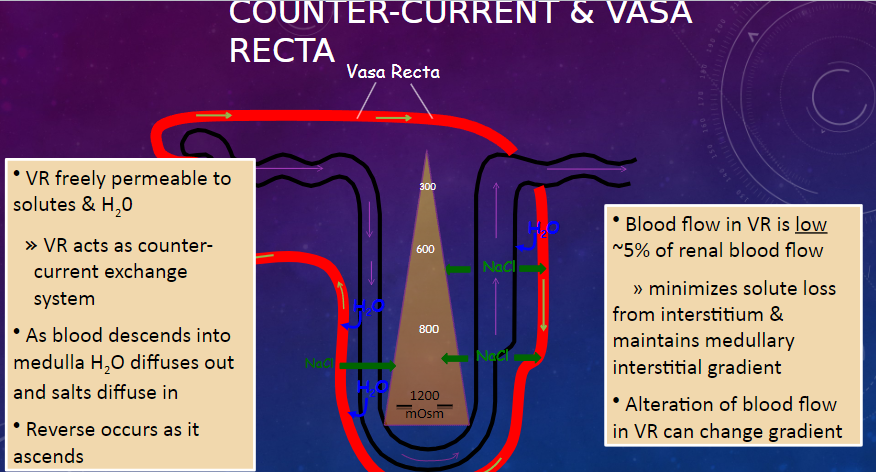
𖹭 The vasa recta (VR) is freely permeable to solutes and water, allowing it to act as a counter-current exchange system.
-
Describe the process of water and salt movement in the vasa recta as blood descends into the medulla and ascends. (2)
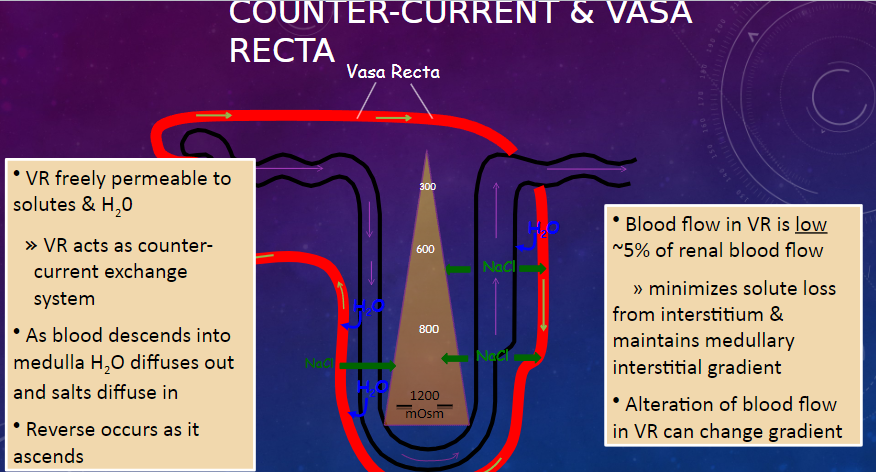
𖹭 As blood descends into the medulla, water diffuses out, and salts diffuse in.
𖹭 The reverse occurs as blood ascends.
-
What is the significance of the low blood flow in the vasa recta? (2)
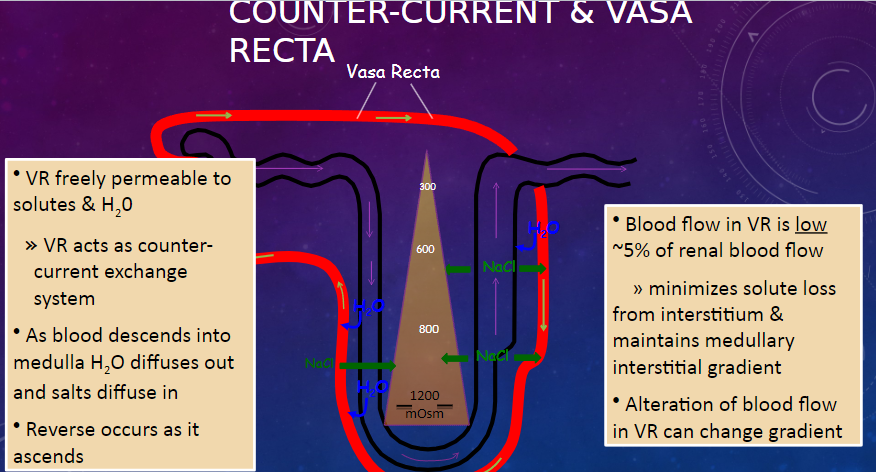
𖹭 Blood flow in the vasa recta is low, approximately 5% of renal blood flow.
𖹭 This minimizes solute loss from the interstitium and maintains the medullary interstitial gradient.
-
How can alteration of blood flow in the vasa recta affect the medullary interstitial gradient? (1)
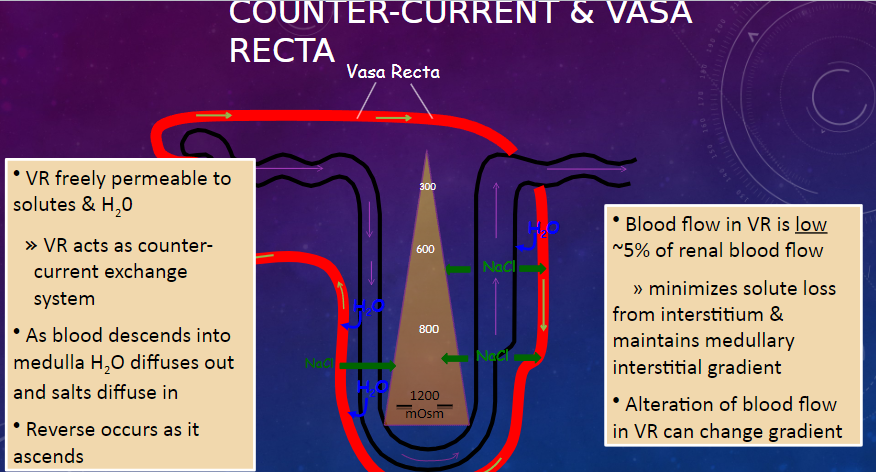
𖹭 Alteration of blood flow in the vasa recta can change the medullary interstitial gradient.
-
What is the significance of the early (first part) distal convoluted tubule? (2)
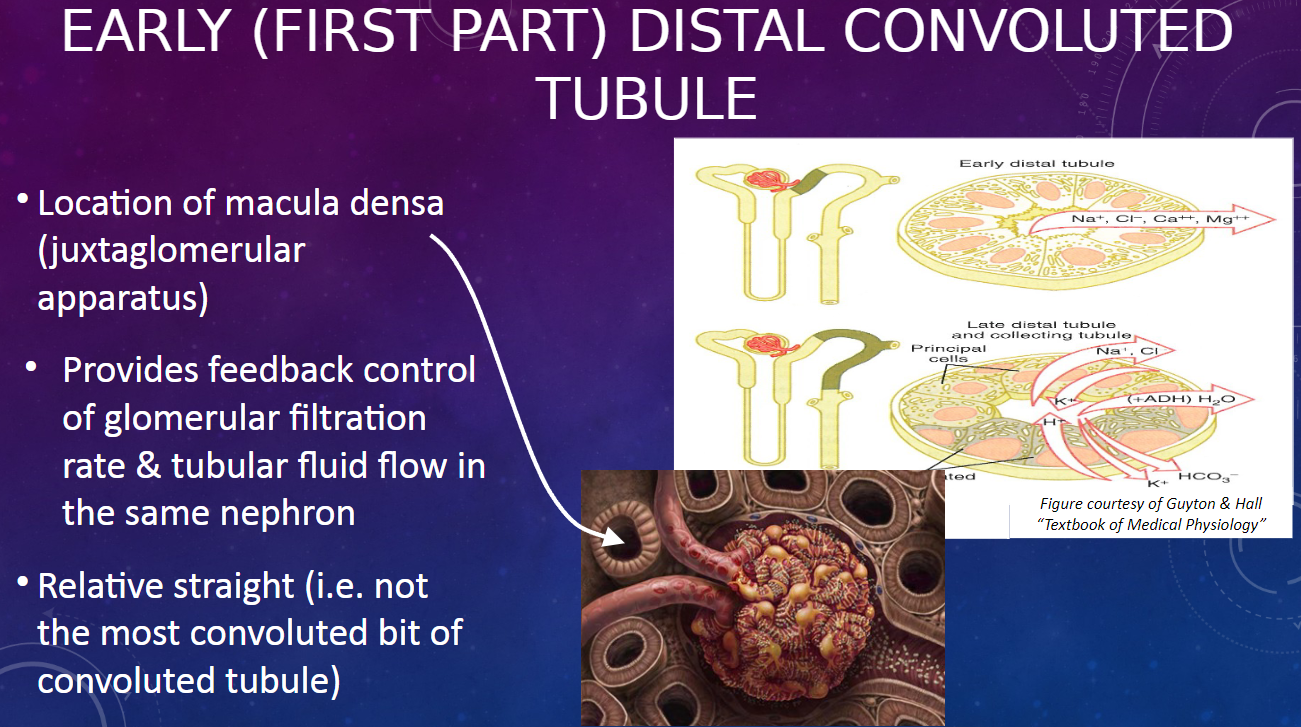
𖹭 It is the location of the macula densa, part of the juxtaglomerular apparatus.
𖹭 It provides feedback control of glomerular filtration rate and tubular fluid flow in the same nephron.
-
Describe the structural characteristic of the early distal convoluted tubule. (1)
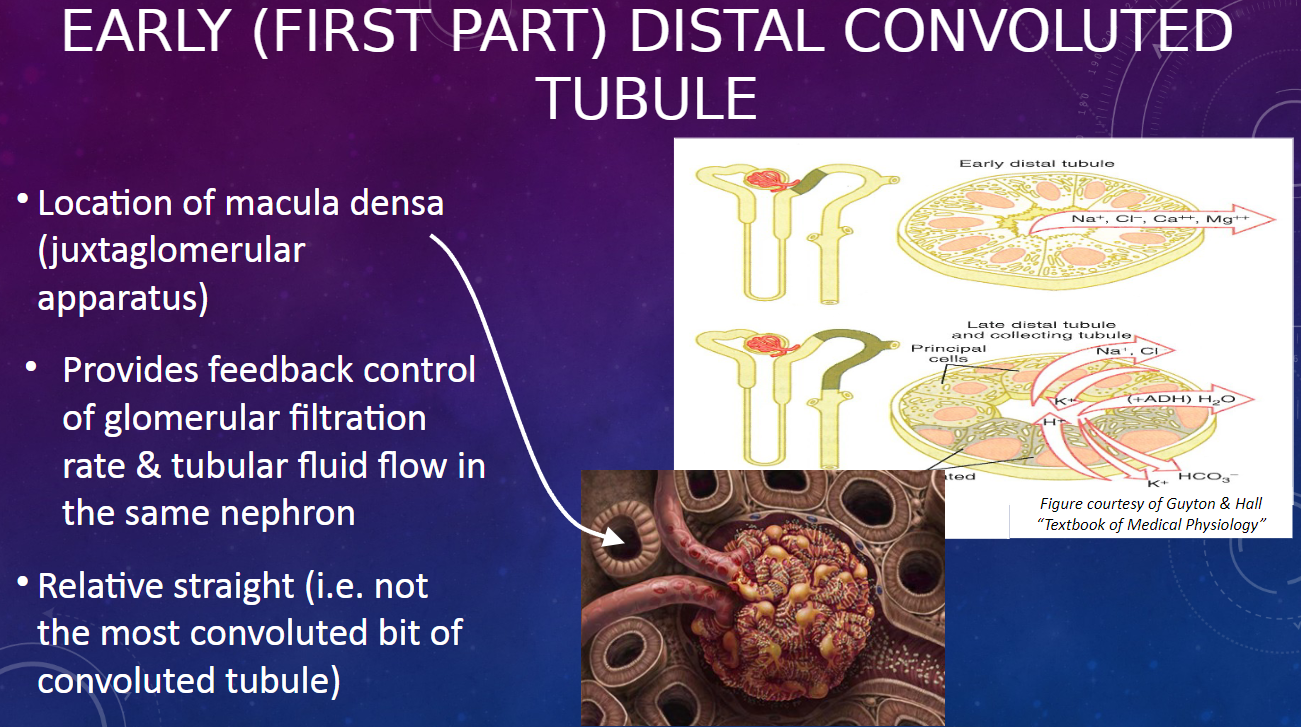
𖹭 The early distal convoluted tubule is relatively straight, unlike the most convoluted parts of the convoluted tubule.
-
What happens in the late (2nd part) distal convoluted tubule regarding solute and water reabsorption? (2)
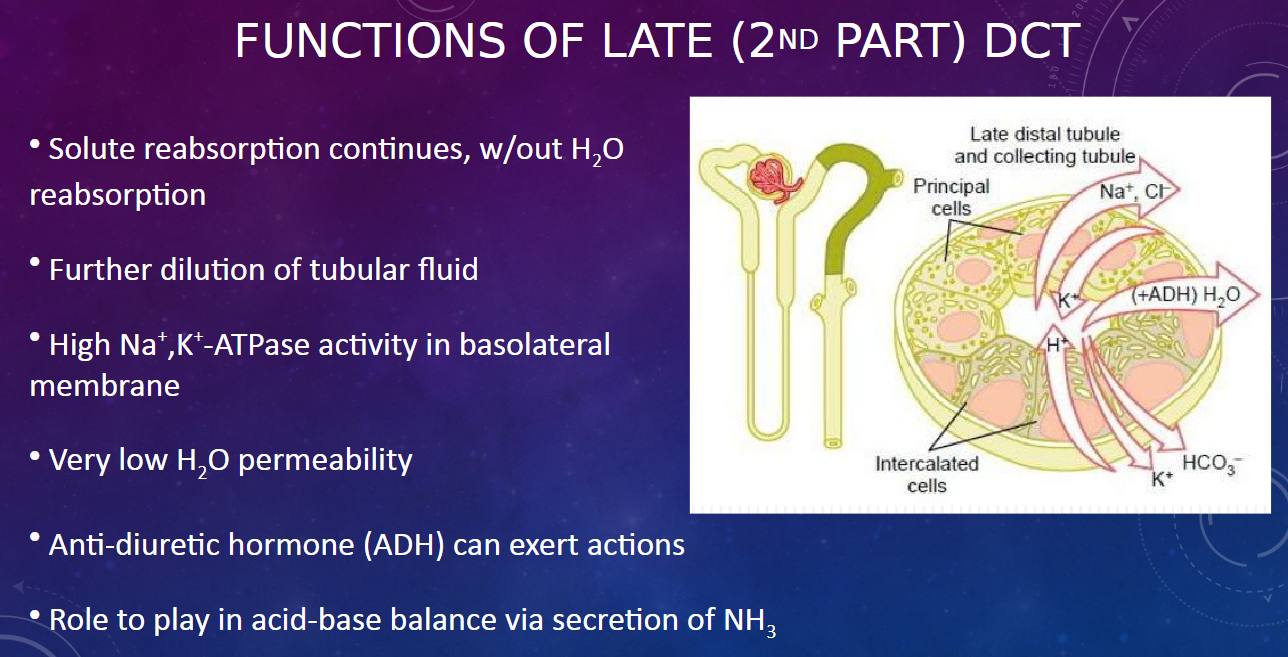
𖹭 Solute reabsorption continues without water reabsorption.
𖹭 This results in further dilution of the tubular fluid.
-
What is the significance of high Na+,K+-ATPase activity in the basolateral membrane of the late DCT? (1)
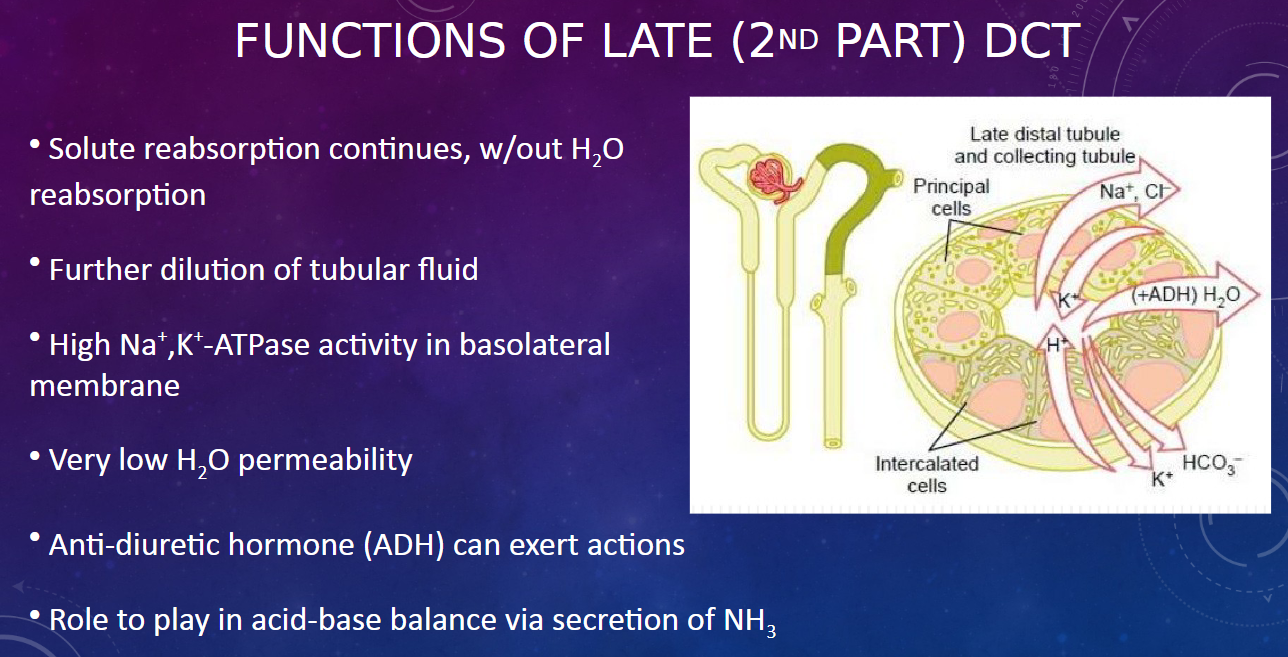
𖹭 The high Na+,K+-ATPase activity maintains ion gradients necessary for solute reabsorption.
-
What is the water permeability like in the late DCT? (1)
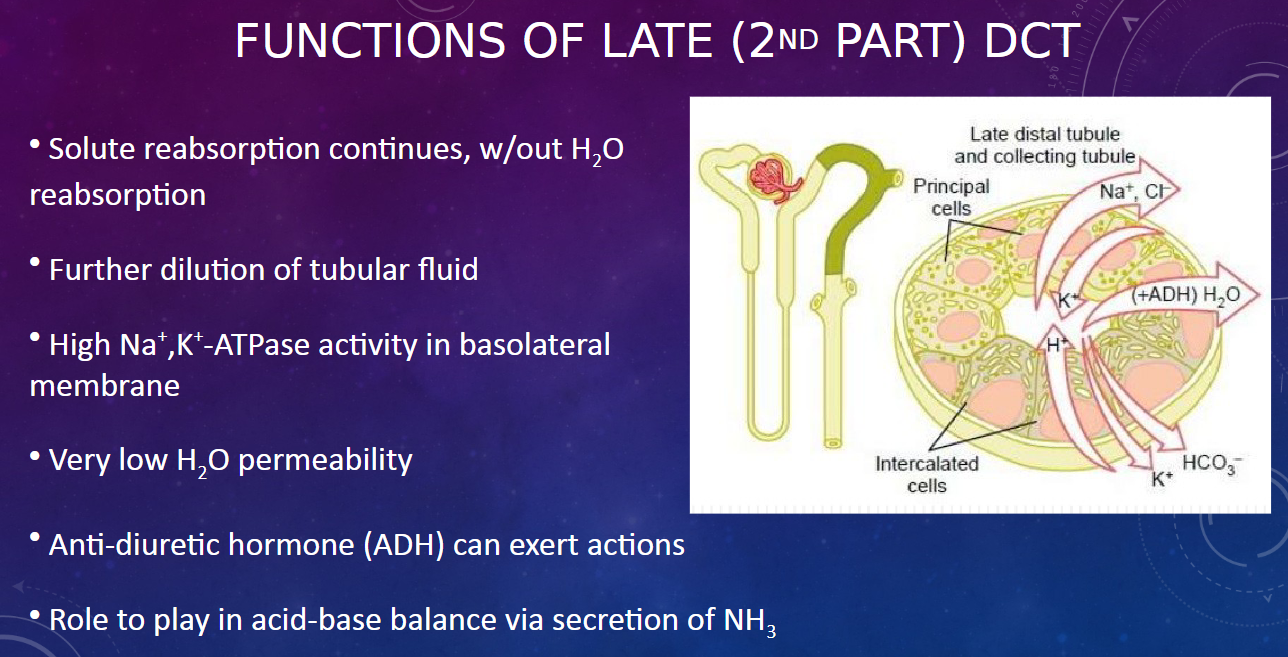
𖹭 The late DCT has very low water permeability, indicating minimal water reabsorption.
-
How does anti-diuretic hormone (ADH) affect the late DCT? (1)
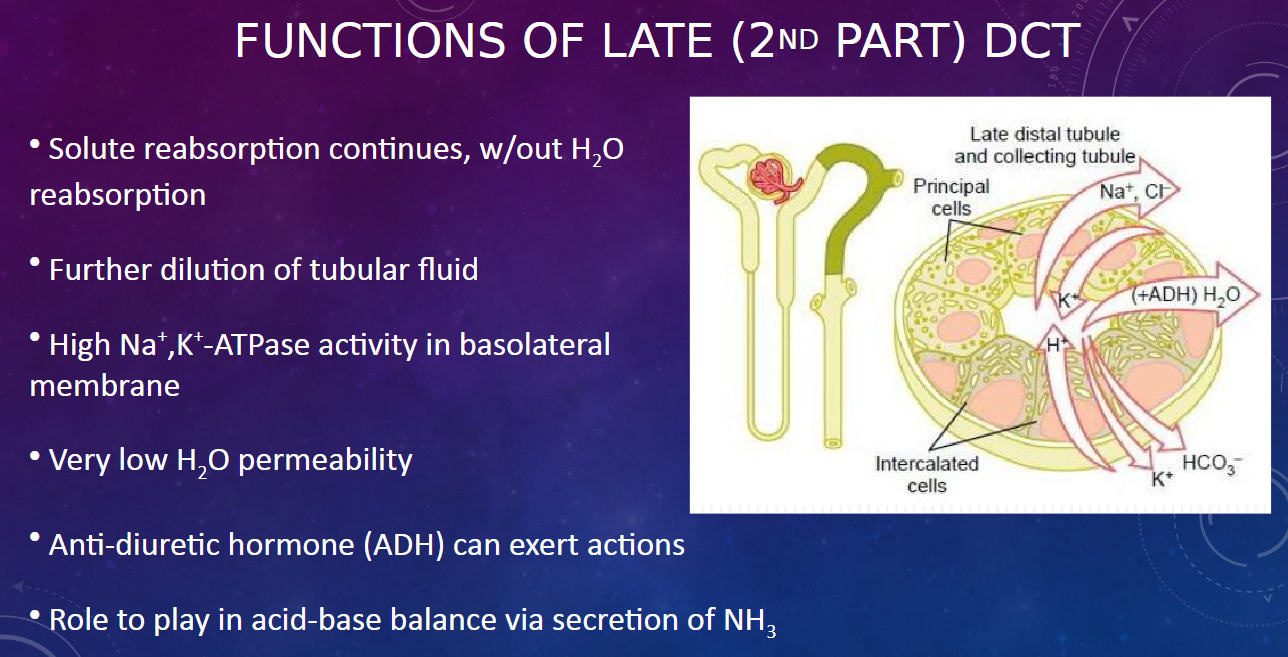
𖹭 ADH can exert actions on the late DCT, influencing water reabsorption based on the body's hydration status.
-
What role does the late DCT play in acid-base balance? (1)
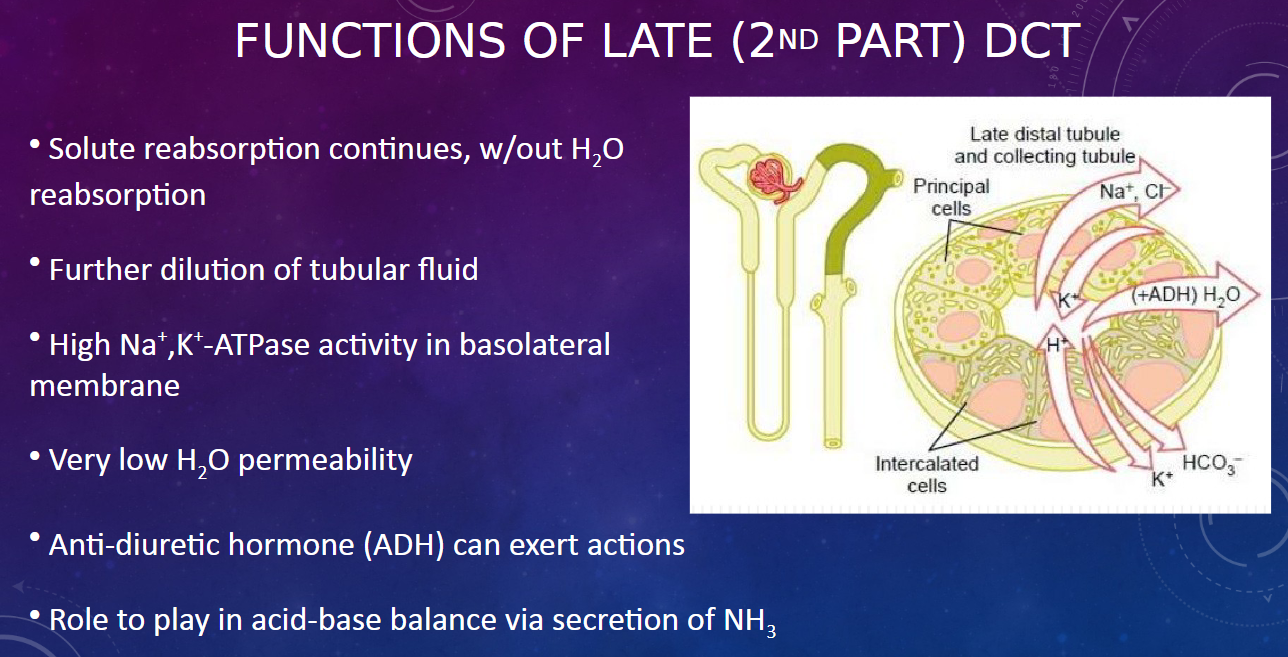
𖹭 The late DCT participates in acid-base balance through the secretion of ammonia (NH3).
-
What is the anatomical location of the collecting (or connecting) tubule? (1)
𖹭 The collecting (or connecting) tubule connects the end of the distal convoluted tubule (DCT) to the collecting duct, mainly in the outer cortex of the kidney.
-
Describe the shape of the collecting (or connecting) tubule. (1)
𖹭 The collecting (or connecting) tubule is relatively straight in shape.
-
How does the collecting (or connecting) tubule compare in functional characteristics with the late DCT and collecting duct? (1)
𖹭 The collecting (or connecting) tubule shares functional characteristics with both the late DCT and collecting duct, indicating overlap in their roles and functions.
-
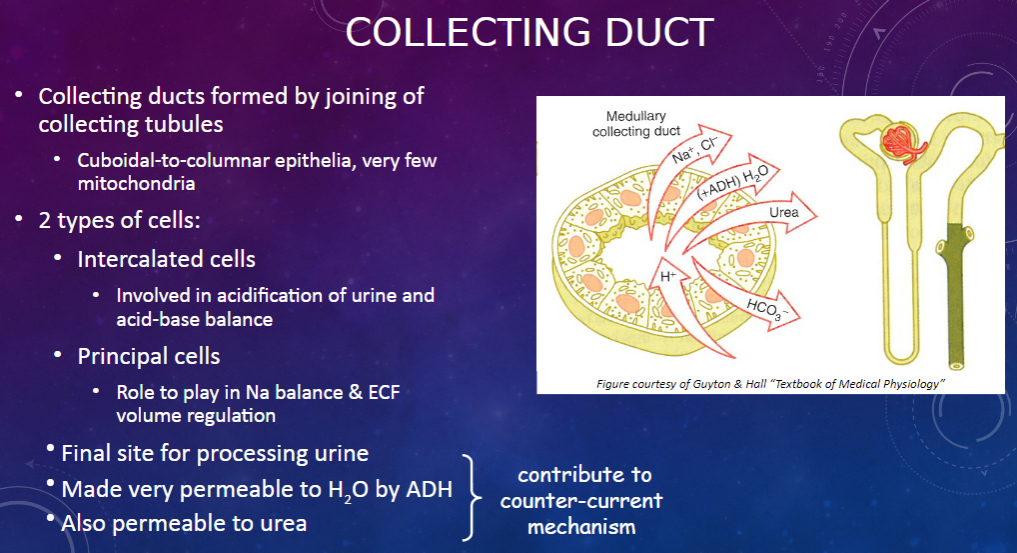
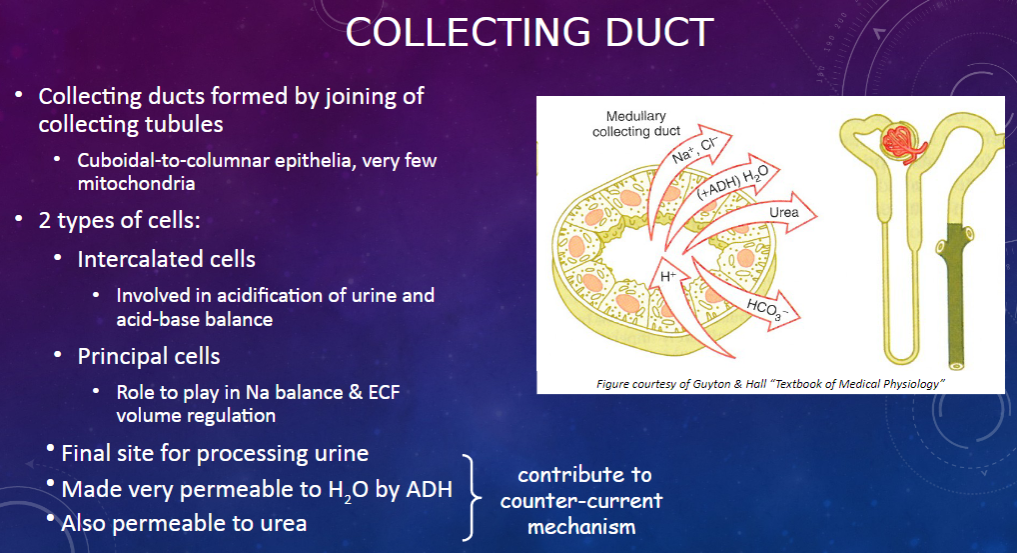
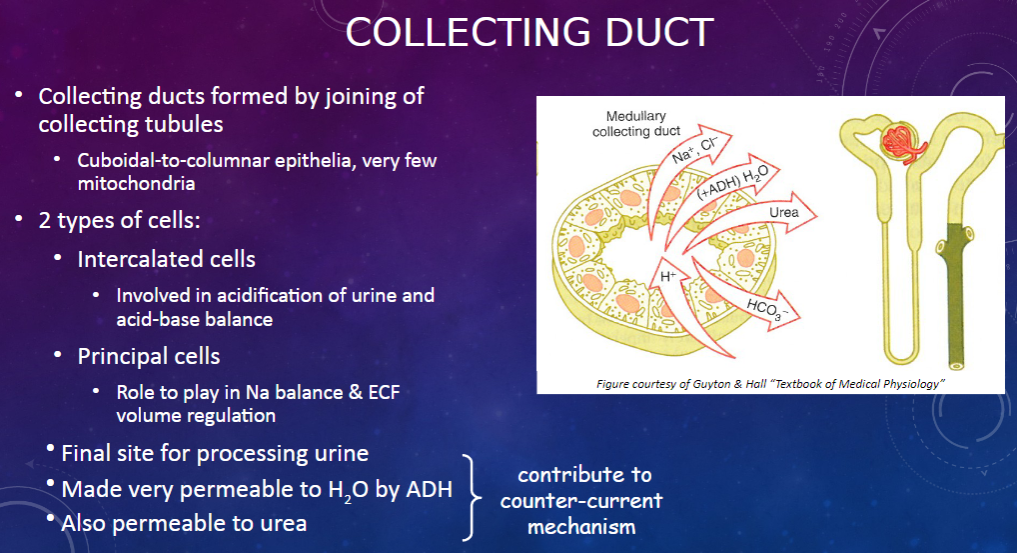
How are collecting ducts formed, and what is their composition? (2)
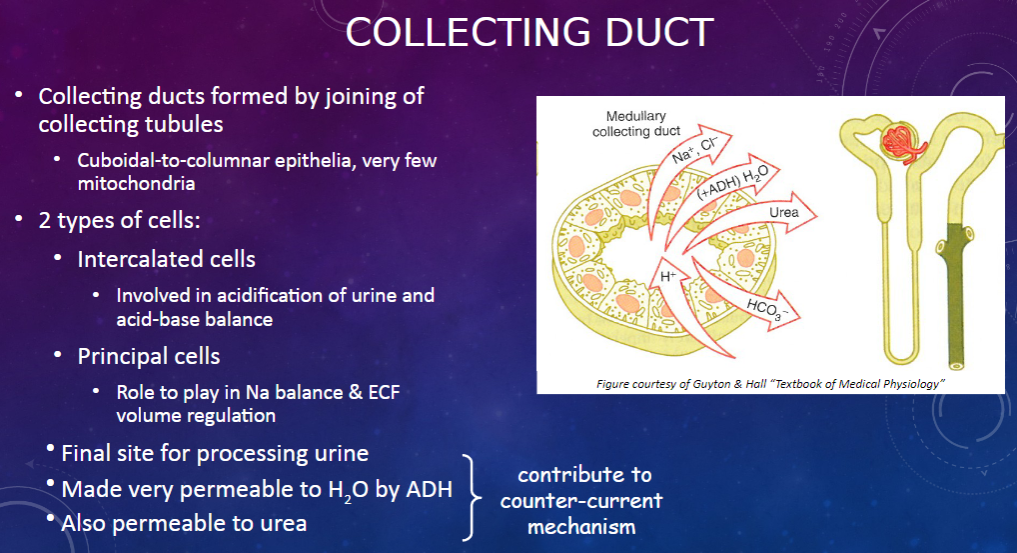
𖹭 Collecting ducts are formed by the joining of collecting tubules.
𖹭 They are composed of cuboidal-to-columnar epithelia with very few mitochondria.
-
What are the two types of cells found in the collecting duct, and what are their functions? (2)
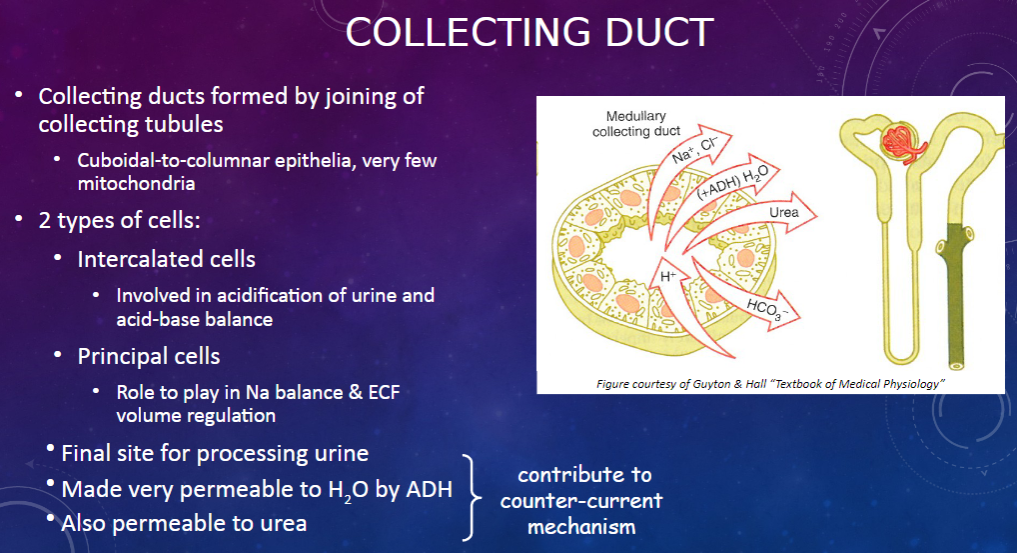
𖹭 Intercalated cells are involved in the acidification of urine and acid-base balance.
𖹭 Principal cells play a role in sodium balance and extracellular fluid (ECF) volume regulation.
-
What is the collecting duct's role in urine processing? (1)
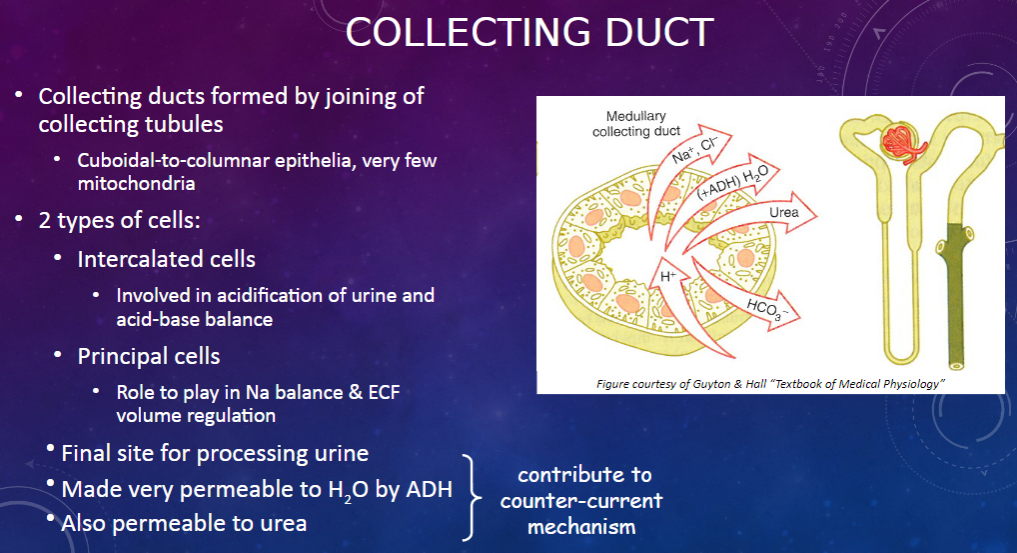
𖹭 The collecting duct serves as the final site for processing urine.
-
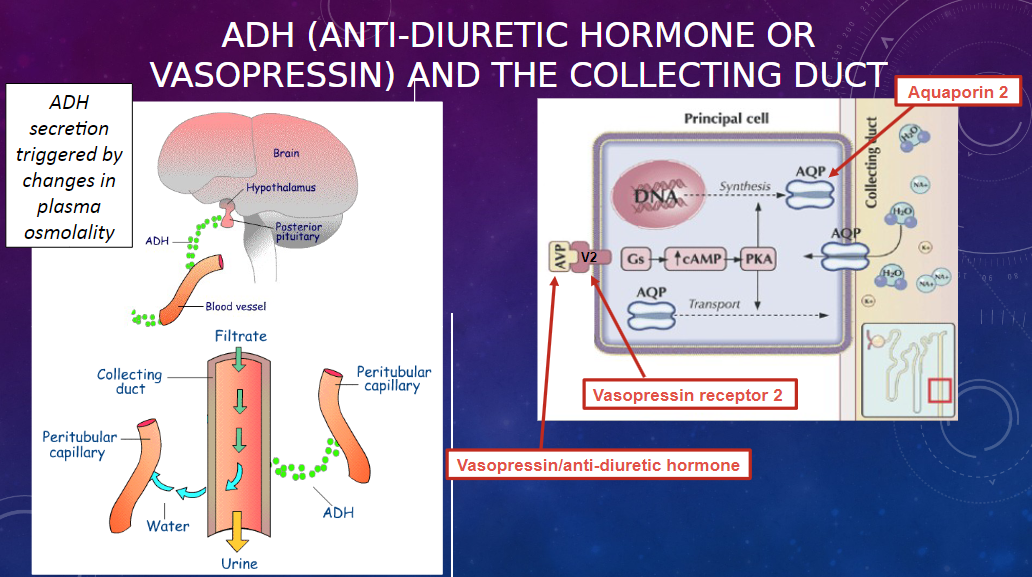
How does the collecting duct respond to anti-diuretic hormone (ADH)? (1)
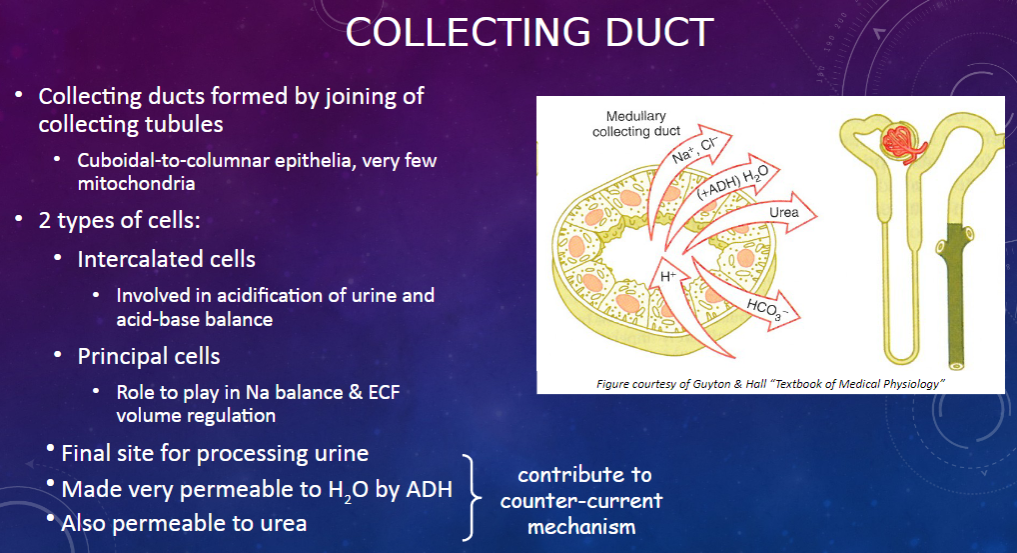
𖹭 ADH makes the collecting duct very permeable to water, facilitating water reabsorption.
𖹭 Additionally, the collecting duct is permeable to urea, contributing to the counter-current mechanism.
-
Picture demonstrating the action of ADH at the collecting duct:
-
Picture demonstrating the functions of the collecting duct:
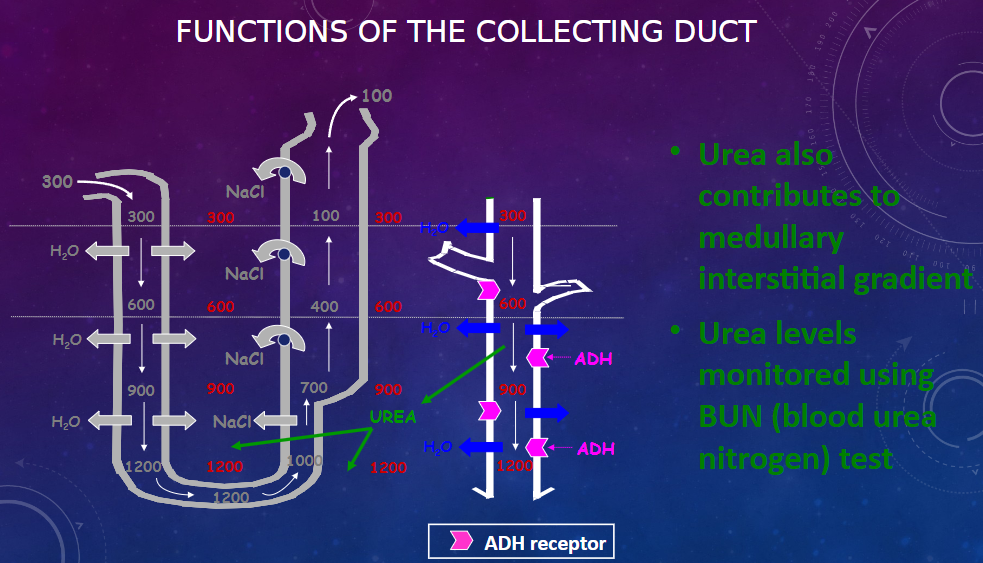
-
Picture demonstrating ADH, DIURESIS AND antidiuresis:
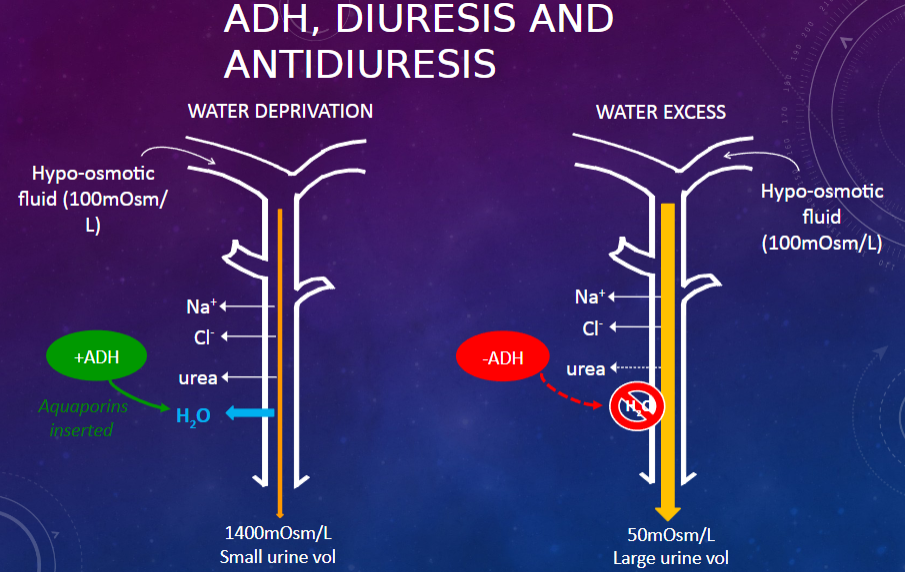
-
What is the first factor contributing to the buildup of solute concentration in the renal medulla? (1)
𖹭 Active transport of Na+ and co-transport of K+ & Cl- out of the thick ascending limb into the medullary interstitium.
-
What is the second factor contributing to the buildup of solute concentration in the renal medulla? (1)
𖹭 Active transport of ions from the collecting ducts into the medullary interstitium.
-
What is the third factor contributing to the buildup of solute concentration in the renal medulla? (1)
𖹭 Facilitated diffusion of large amounts of urea from the collecting ducts into the medullary interstitium.
-
What is the fourth factor contributing to the buildup of solute concentration in the renal medulla? (1)
𖹭 Very little diffusion of water from the ascending limbs of tubules into the medullary interstitium.
-
What is Polycystic Kidney Disease (PKD), and how is it characterized? (2)
𖹭 Polycystic kidney disease is a genetic disorder characterized by the growth of numerous cysts within the kidneys.
𖹭 It is characterized by the growth of numerous cysts within the kidneys.
-
What is Glomerulonephritis (GN), and what are its primary characteristics? (2)
𖹭 Glomerulonephritis involves inflammation of the glomeruli in some or all of the million nephrons in the kidney.
𖹭 It can be primary or secondary to systemic diseases like diabetes mellitus.
-
Describe the types of diseases affecting the tubule. (1)
𖹭 Diseases of the tubule can involve obstruction, reducing glomerular filtration, or impairment of transport functions, reducing water and solute reabsorption. An example is Fanconi’s syndrome.
-
What are the acquired kidney diseases discussed? (4)
𖹭 Hypertension: The kidneys regulate extracellular fluid volume and hence influence blood pressure. Compensatory mechanisms in response to high blood pressure can lead to chronic kidney damage.
𖹭 Congestive Cardiac Failure: A fall in cardiac output leads to renal hypoperfusion, registered as hypovolemia. Compensation results in pulmonary edema.
𖹭 Diabetic Nephropathy: As a consequence of diabetes (poor glucose control and hypertension), the filtering system of the kidneys gets destroyed over time.
𖹭 Lithium Treatment Resulting in Acquired Nephrogenic Diabetes Insipidus: Lithium treatment can result in acquired nephrogenic diabetes insipidus due to a reduction of AQP2 expression. Nephrogenic diabetes insipidus (NDI) is a condition characterized by kidney or nephron dysfunction, leading to insensitivity to antidiuretic hormone (ADH), resulting in excessive thirst and excretion of large amounts of severely diluted urine.

