-
Alkali Metals (group)group 1 on the periodic table.
-
Alkaline Earth Metals (group)group 2 on the periodic table.
-
Transition Metals (group)groups 3-12.
-
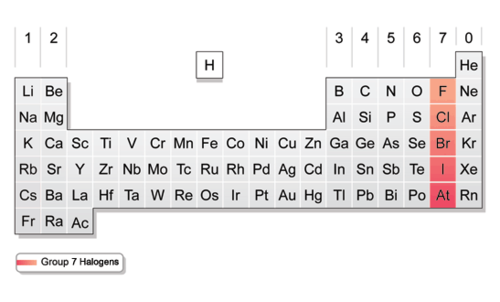
Halogens (group)group 17.
-
valence electronsare found in the outermost energy level.
-
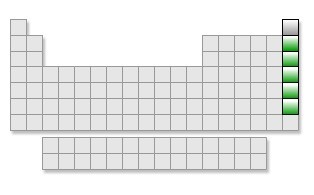
Noble Gases (group)group 18.
-
Electronegativitytendency of an atom to attract/gain electrons
-
Ionization Energythe amount of energy required to remove an electron.
-
Atomic Radius1/2 the distance between nuclei of the same element bonded together.
-
Alkaline Earth Metals (group)group 2.
-
Metalslose electrons/form cations.
-
Non Metalsgain electrons/form anions.
-
Orbitalregion of space where electrons are most likely to be found.
-
Electron (Lewis) Dot Diagram.
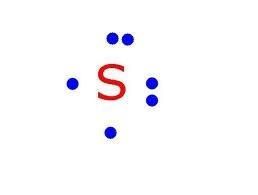 Shows valence electrons.
Shows valence electrons. -
Periods/Rows.From left to right across the periodic table.
-
Alkaline Earth Metals (v.e.)two valence electrons.
-
Halogens (v.e.)seven valence electrons.
-
Noble Gases (v.e)8 valence electrons/ least reactive.
-
Noble Gaseshigh ionization energy/ NO electronegativity.
-
metalsmost of the elements on the periodic table.
-
Electronegativity Trend Across A Period goes (up/down)goes up left to right across period/ row
-
Electronegativity Trend Down a Group goes (up/down)goes down while going down the group/column/family
-
Ionization energy Trend Across A Period (increase/decrease)increases left to right across period/row.
-
Ionization Energy Trend Down A Group (increase/decrease)decreases going down the group/column/family.
-
Atomic Radius Trend Across A Perioddecrease right to left across the period/row..
-
Atomic Radius Trend Down A Groupincreases going down the group/column/family
-
Ga Al SiGa
-
Ca Mg SrMg
-
Cl S PP
-
P, S, Cl,Cl
-
N K HeHe
-
N K He Na CaK
-
Li, Be, Mg, NaNa
-
Br As KAs
-
He F I ClF
-
C N OC
-
An increase in distance between a proton and electron (increase/ decrease) the force.decrease (force of attraction)
-
As orbitals are added down a group how does the attractive force between valence electrons and the nucleus charge change? (Increase or Decrease)Decrease (force of attraction)
-
The attractive force (increase/ decrease) as number of protons in the nucleus increases.Inrease (force of attraction)
-
Halogens (picture)
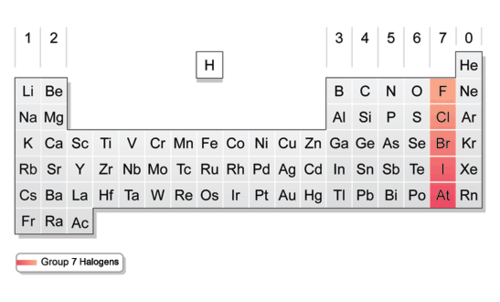
-
Alkali Metals (picture)
-
Alkaline Earth Metals (picture)
-
Metalloids (picture)

-
Non Metals (picture)
-
Transition Metals (picture)
-
Noble Gases (Picture)