Alkali Metals (group)
group 1 on the periodic table.
Alkaline Earth Metals (group)
group 2 on the periodic table.
Transition Metals (group)
groups 3-12.
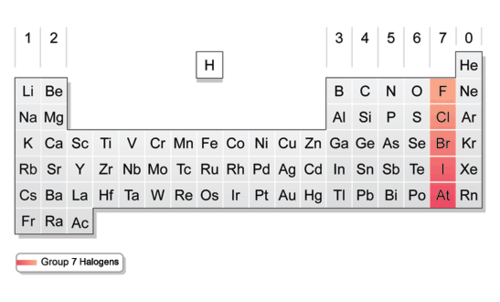
Halogens (group)
group 17.
valence electrons
are found in the outermost energy level.
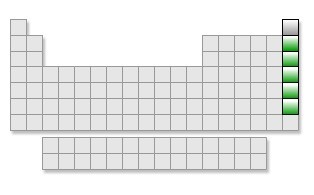
Noble Gases (group)
group 18.
Electronegativity
tendency of an atom to attract/gain electrons
Ionization Energy
the amount of energy required to remove an electron.
Atomic Radius
1/2 the distance between nuclei of the same element bonded together.
Alkaline Earth Metals (group)
group 2.
Metals
lose electrons/form cations.
Non Metals
gain electrons/form anions.
Orbital
region of space where electrons are most likely to be found.
Electron (Lewis) Dot Diagram.
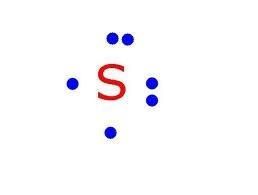
Shows valence electrons.
Periods/Rows.
From left to right across the periodic table.
Alkaline Earth Metals (v.e.)
two valence electrons.
Halogens (v.e.)
seven valence electrons.
Noble Gases (v.e)
8 valence electrons/ least reactive.
Noble Gases
high ionization energy/ NO electronegativity.
metals
most of the elements on the periodic table.
Electronegativity Trend Across A Period goes (up/down)
goes up left to right across period/ row
Electronegativity Trend Down a Group goes (up/down)
goes down while going down the group/column/family
Ionization energy Trend Across A Period (increase/decrease)
increases left to right across period/row.
Ionization Energy Trend Down A Group (increase/decrease)
decreases going down the group/column/family.
Atomic Radius Trend Across A Period
decrease right to left across the period/row..
Atomic Radius Trend Down A Group
increases going down the group/column/family
Ga Al Si
Ga
Ca Mg Sr
Mg
Cl S P
P
P, S, Cl,
Cl
N K He
He
N K He Na Ca
K
Li, Be, Mg, Na
Na
Br As K
As
He F I Cl
F
C N O
C
An increase in distance between a proton and electron (increase/ decrease) the force.
decrease (force of attraction)
As orbitals are added down a group how does the attractive force between valence electrons and the nucleus charge change? (Increase or Decrease)
Decrease (force of attraction)
The attractive force (increase/ decrease) as number of protons in the nucleus increases.
Inrease (force of attraction)
Halogens (picture)
Alkali Metals (picture)
Alkaline Earth Metals (picture)
Metalloids (picture)

Non Metals (picture)
Transition Metals (picture)
Noble Gases (Picture)