-
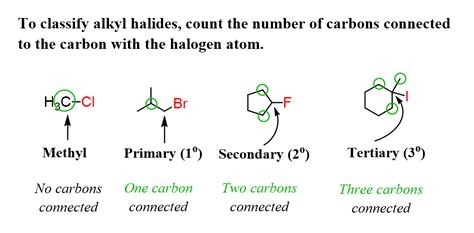
What is methyl, primary, secondary and tertiary?
-
What is an allylic group?
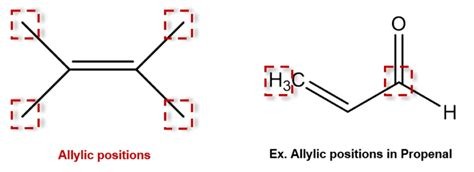
an allylic group is adjacent to a double bond. (allylic chloride, allylic alcohol, allylic hydrogen, etc)
-
What is an benzylic group?
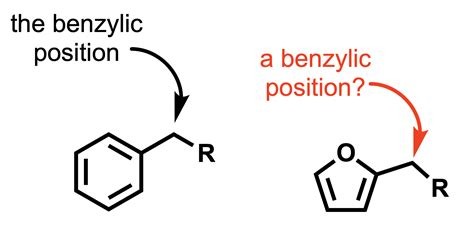
an benzylic group is adjacent to an aromatic ring. (benzylic chloride, benzylic alcohol, benzylic hydrogen, etc)
-
What is vinyl group?
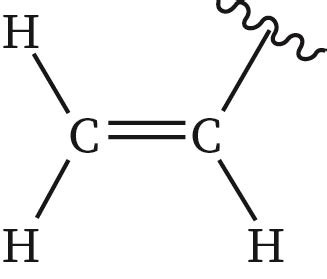
-
What is the hydrolysis reaction?
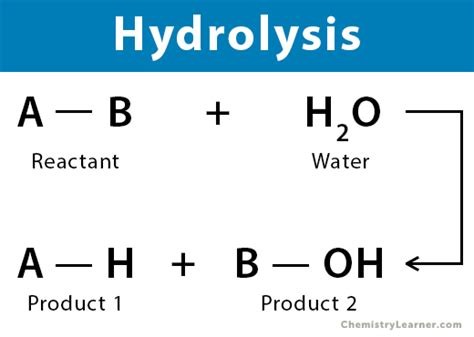
-
What is a solvolysis reaction?
figure it out
-
1) What is the relationship between resonance structures and stability of the intermediary? 2) How does the stability of the intermediary affect the % output of products? 3) How does stability of charge affect energy requirements?
1) Some resonance structures are more stable than others. The stable ones are more likely. 2) if a resonance structure is stable (charge on a tertiary carbon rather than a primary carbon for example), then that resonance structure is more probable. Thus it will react and form a product instead of the less likely resonance structure reacting and forming a product. (see lec2 pg1 notes for visual) 3) Less energy is required for a reaction, if the products of the reaction are stable. Resonance is a stabilizing factor.
-
1) What is deprotonation? 2) What is hydrogen abstraction?
1) Removing a hydrogen where the 2 electrons that bonded the hydrogen & carbon stay with the carbon. 2) Removing a hydrogen where one electron goes with the hydrogen, and the other electron stays with the carbon, forming two radicals.
-
radical mechanism and its indication
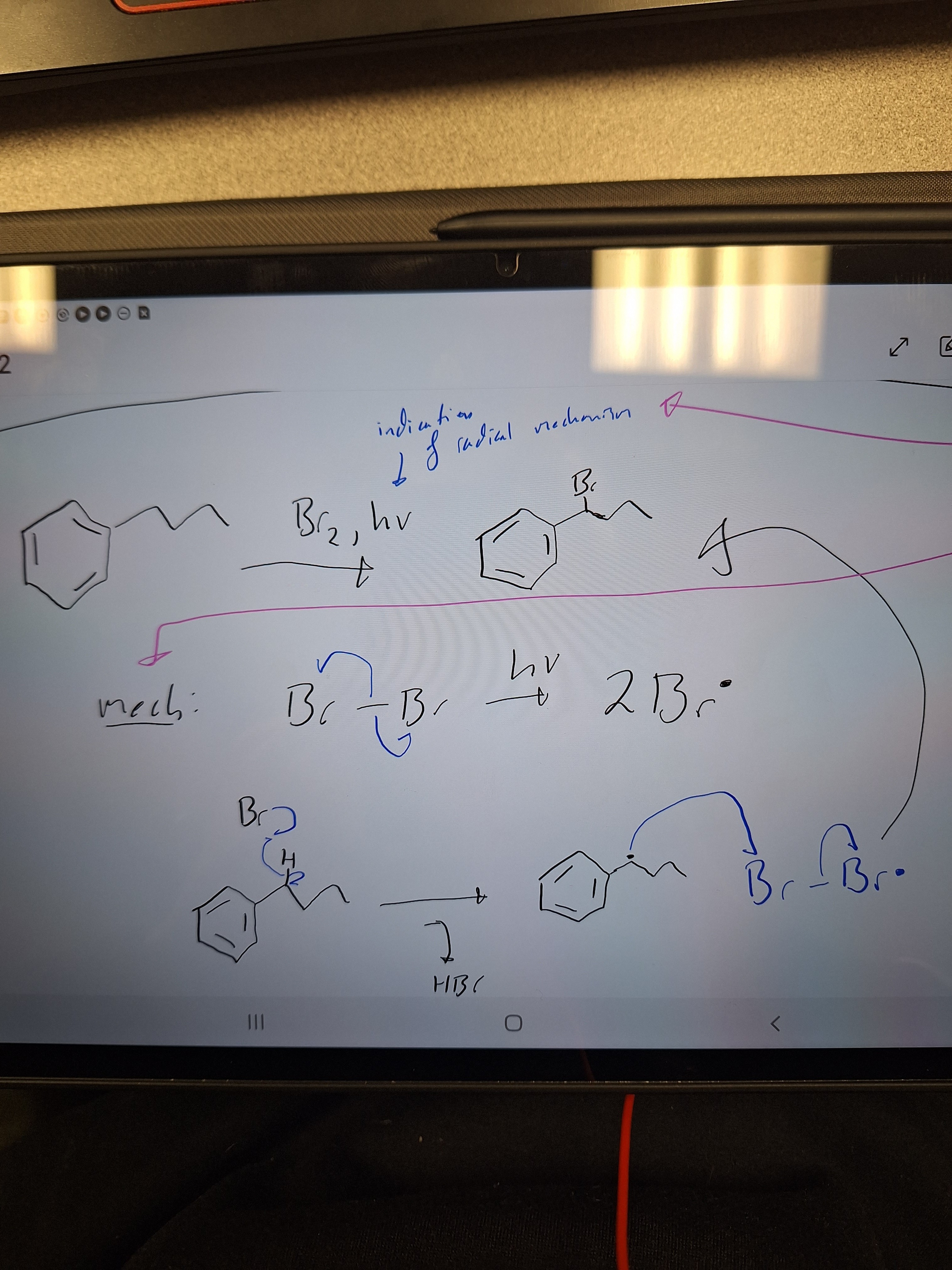
hv (light) is the indication.
-
How does resonance affect acidity?
More possible resonance structures -> more stable charge -> easier to give H away thus more acidic
-
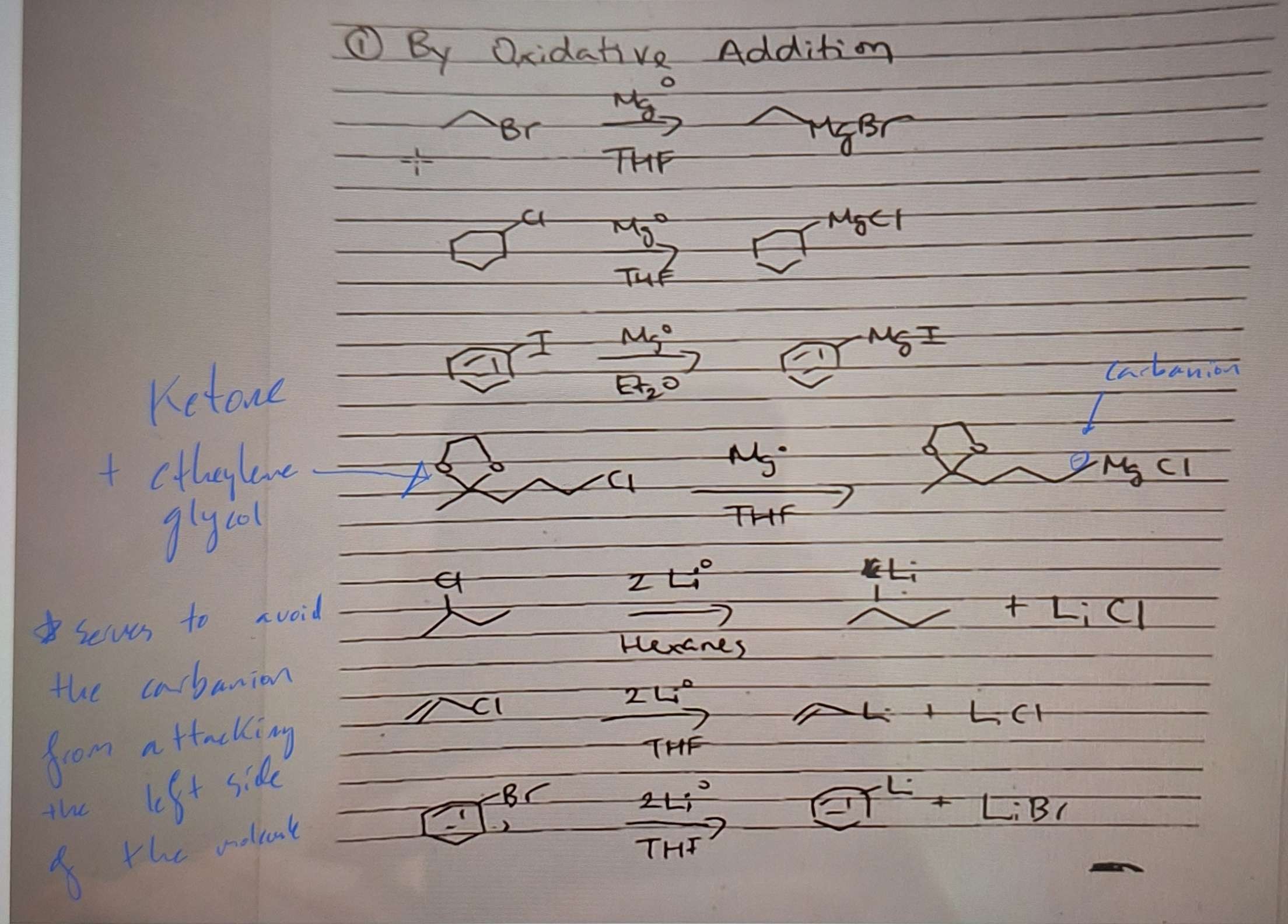
Write out the gignard rxn. Solvent specs? Steps?

-
SN1, SN2, E1, E2 reaction mechs
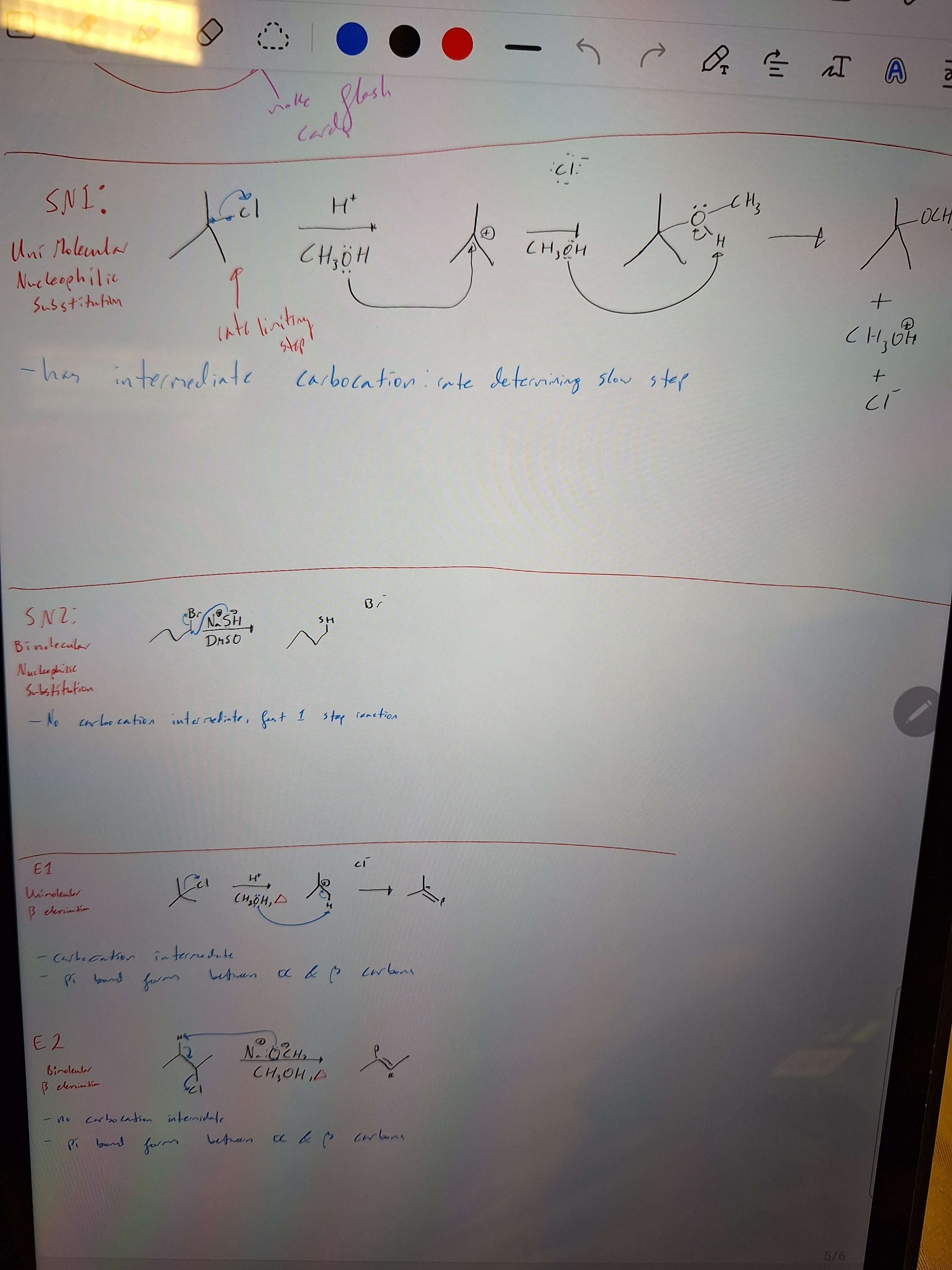
-
Why do acidic beta-hydrogens increase rate of E2 rxn?

-
How do allylic and benzylic vs their counterparts affect SN2 reactivity rate?

-
Allylic/Benzylic oxidation reaction mech
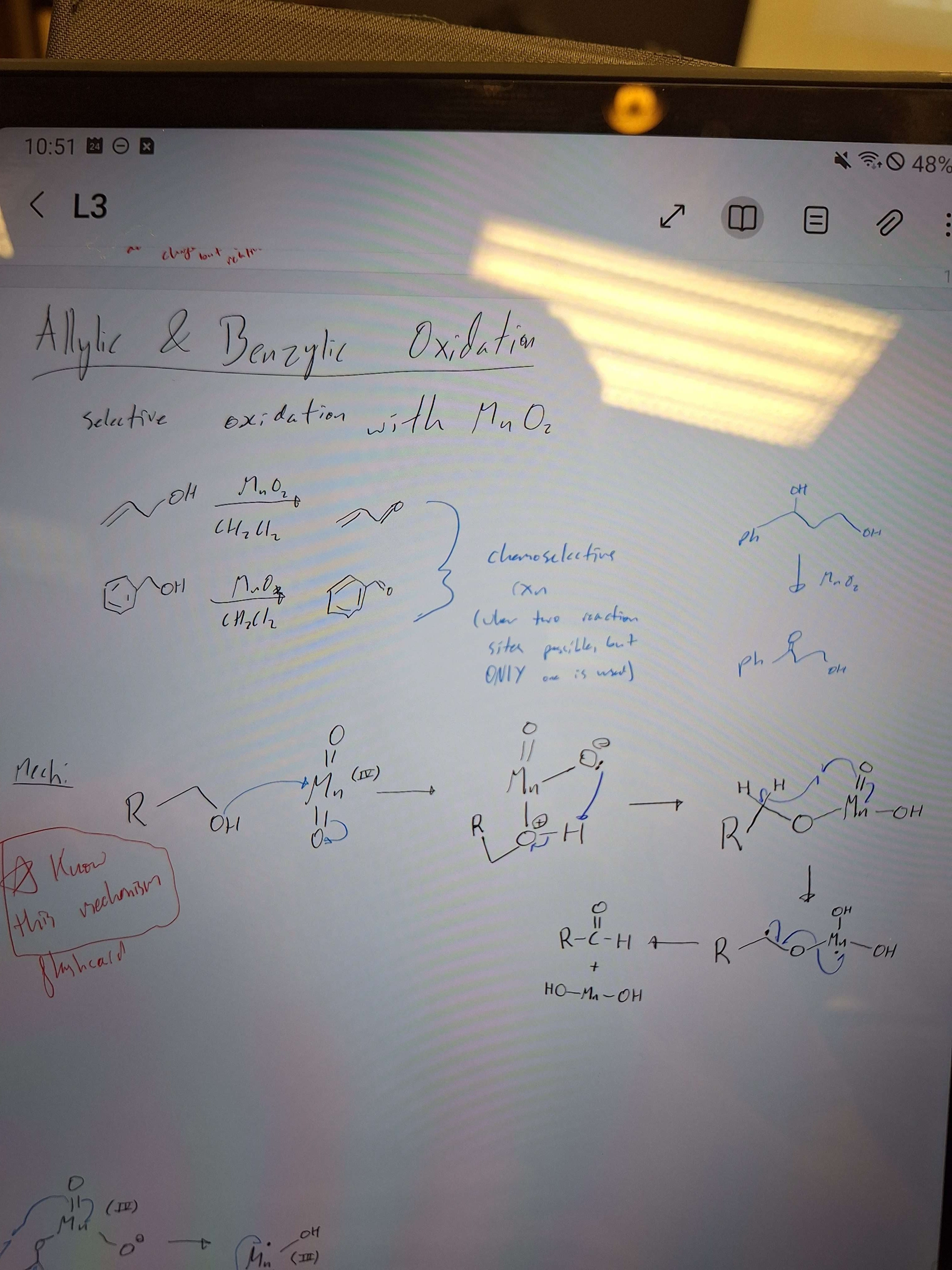
-
What is an aryl/vinyl halide? What is a phenol?
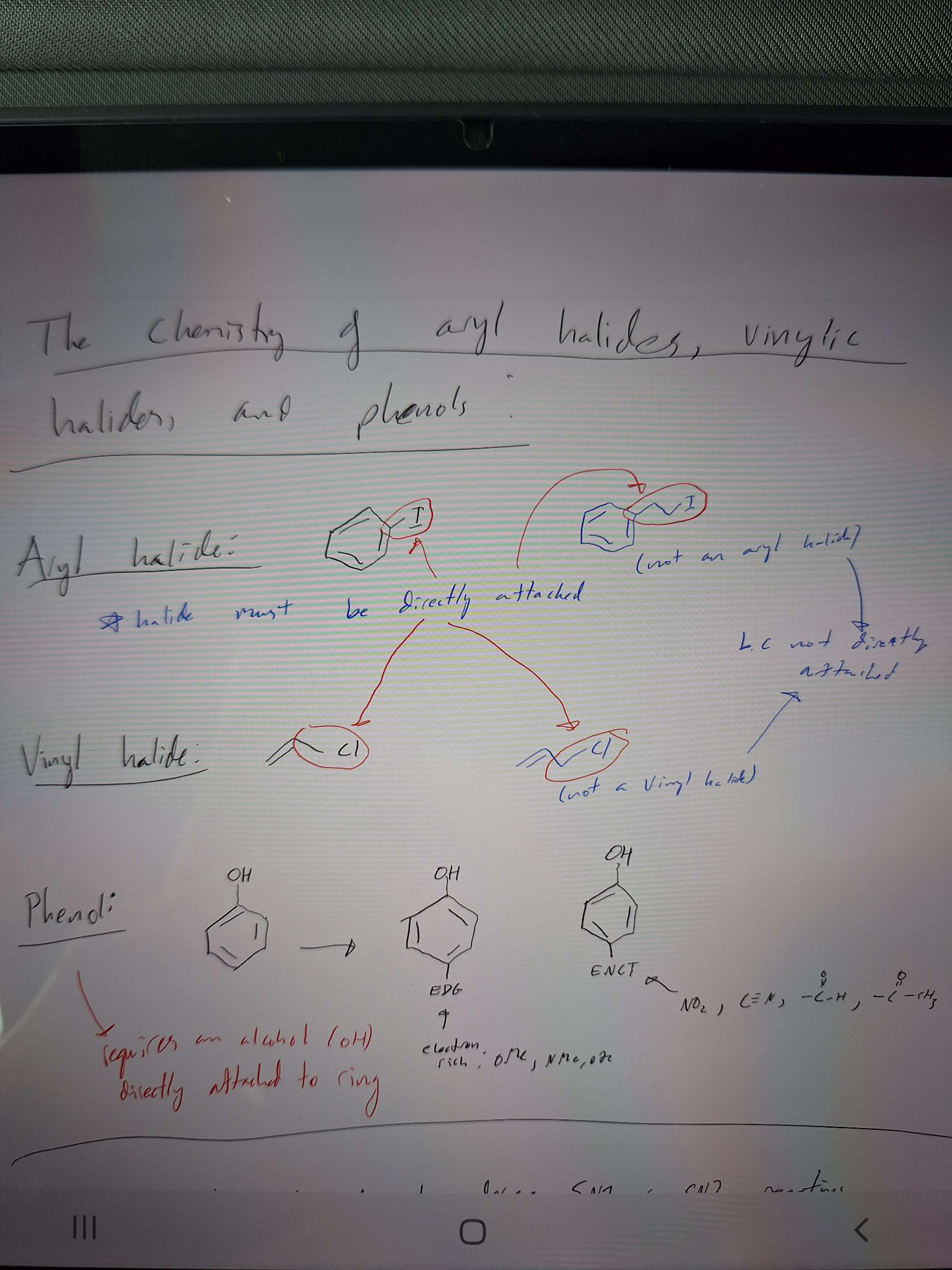
-
Why do Vinyl halides not undergo SN1 SN2 reactions?
Vinyl Halides dont bc the carbon attached to halide is sp2 hybridized, so 33% s character which is strong. Also, going from sp2 to sp for a SN1 or 2 reaction requires alot of energy. Also, this would involve a cation, in a high electron dense area. Not likely!
-
Why do Aryl halides not undergo SN1 SN2 reactions?
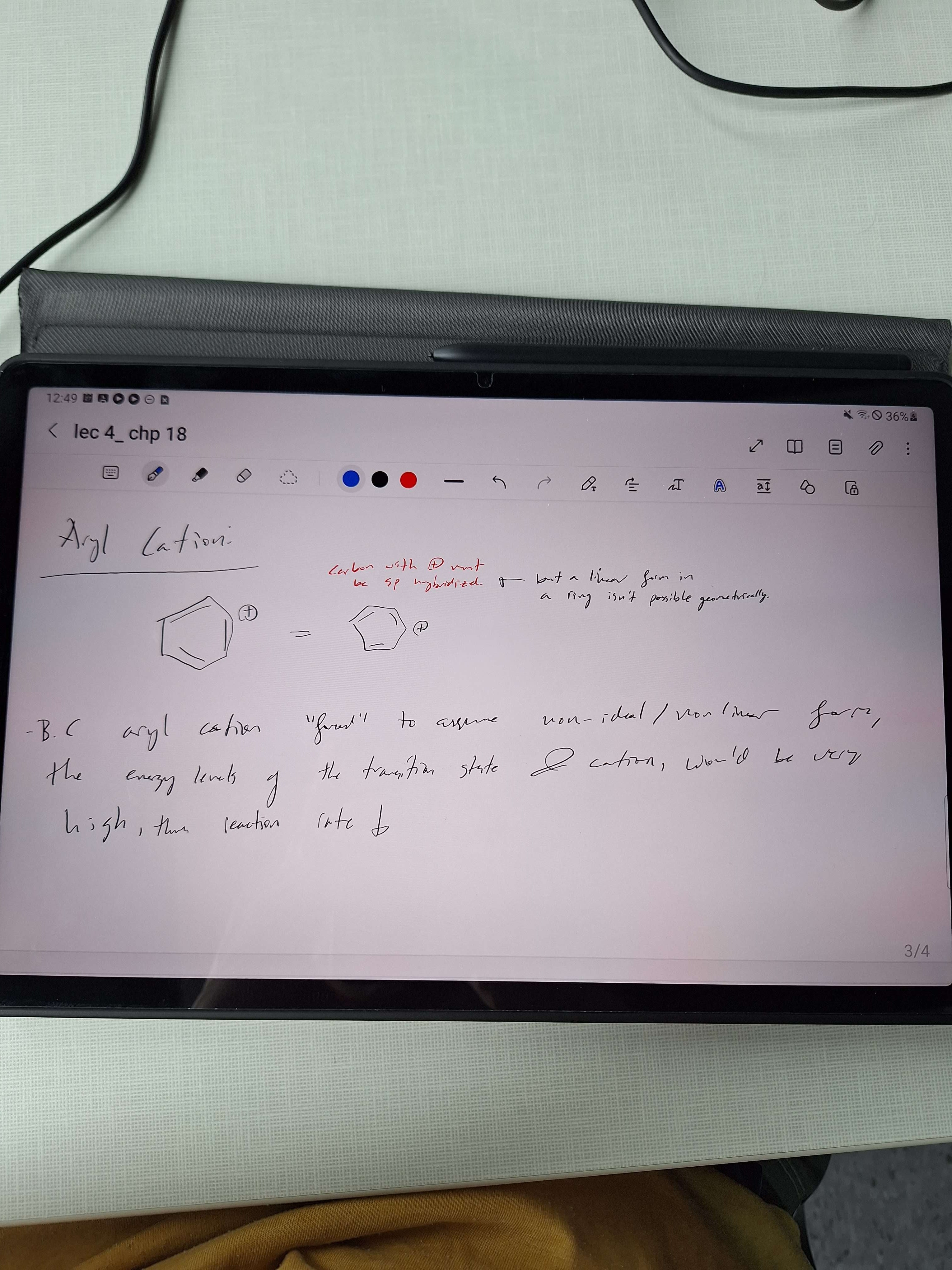
Also, this would involve a cation, in a high electron dense area. Not likely!
-
elimination reactions of vinyl halides

-
EWG (electron withdrawing group) effect of SnAr rate? Which positions have an effect?

meta position cannot resonate/stabilize charge from Nuc. so thus doesnt accelerate reaction
-
Why is Florine the most reactive halogen for SnAr?

-
SnAr mechanism and Meisenheimer complex
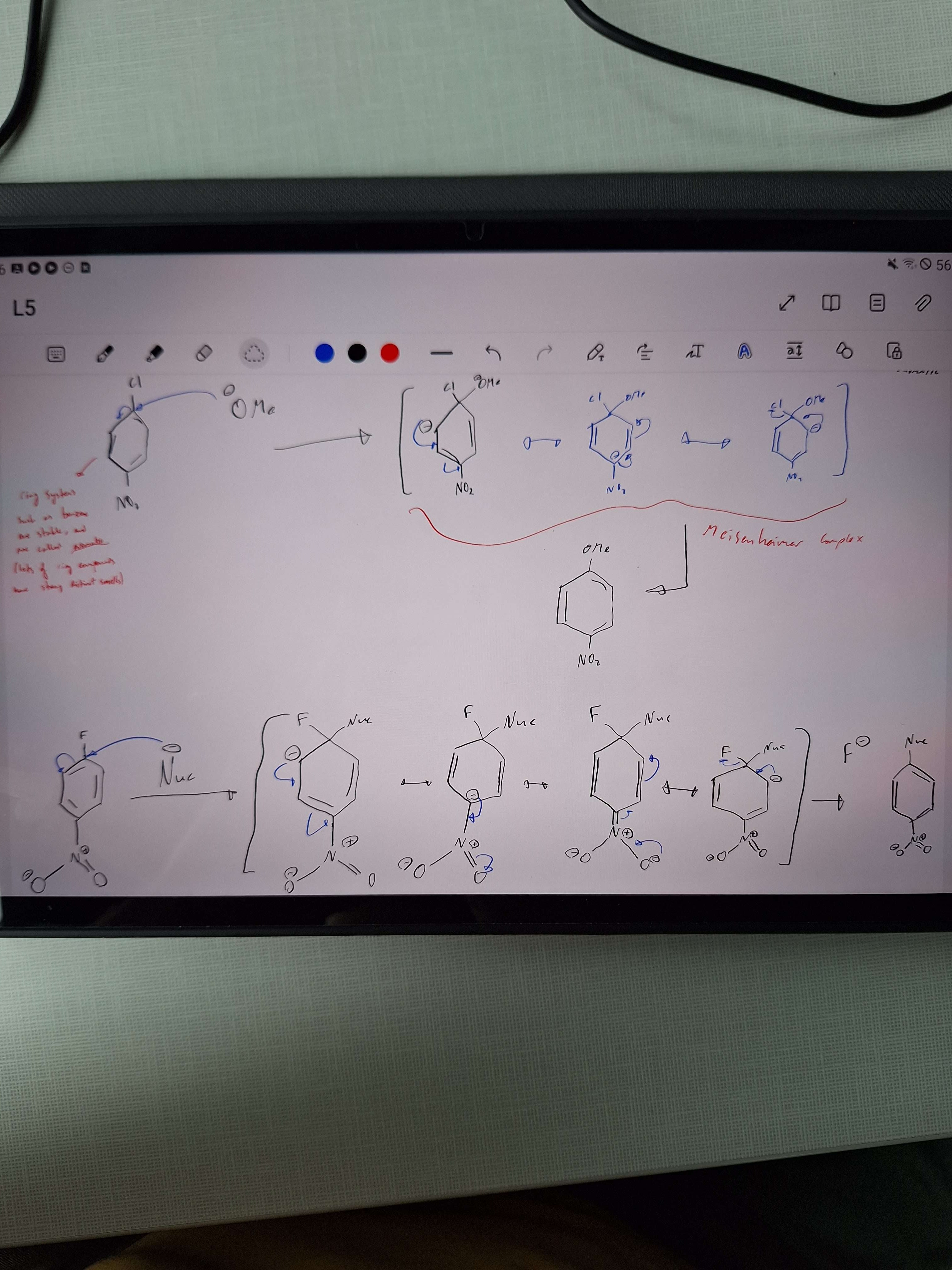
-
The Heck reaction mech
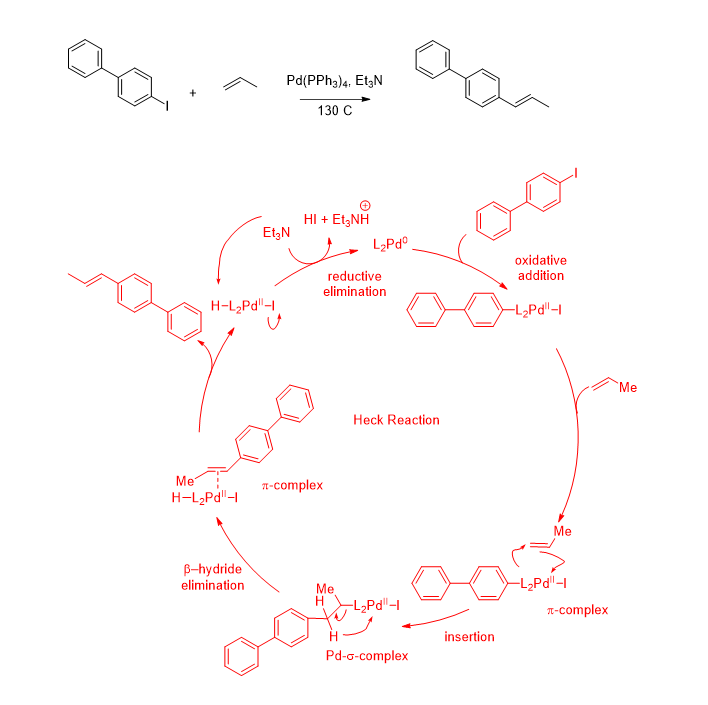
-
Phenol chemistry
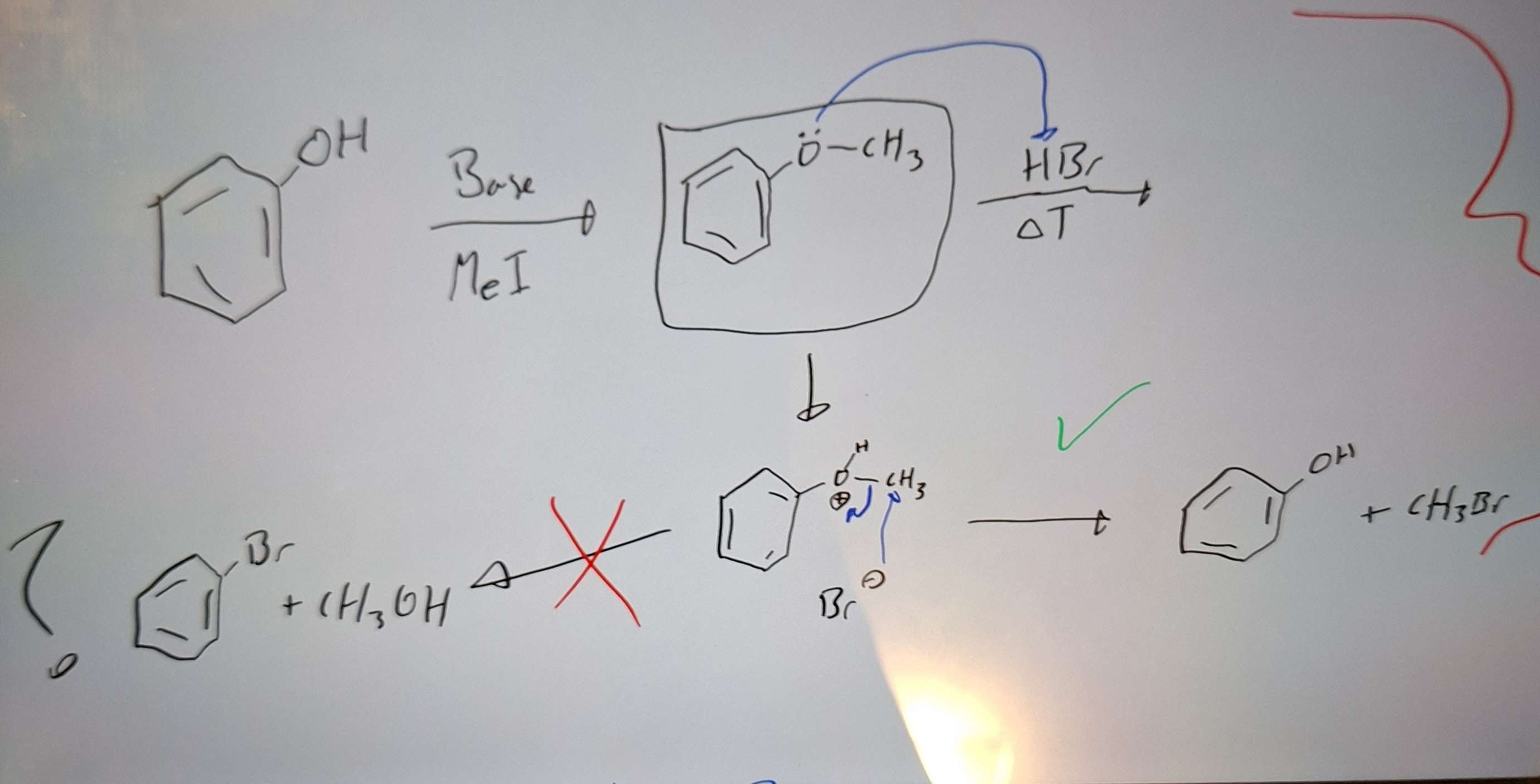
-
Palladium catalyst reactions

-
functional group effects on pKa
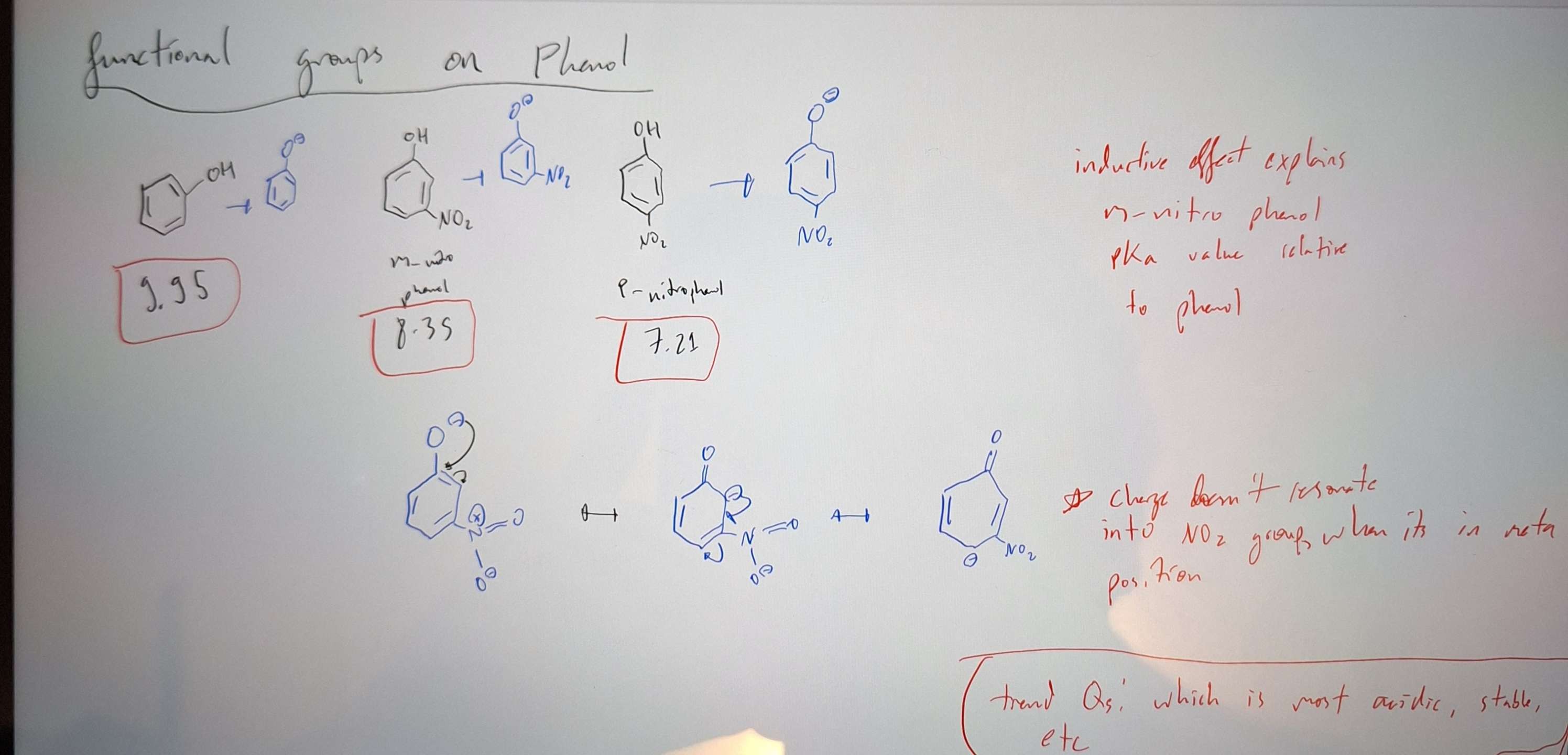
-
Synthesis of Aldehydes and ketones 1) Oxidation of alcohols - you need a hydrogen to remove from the carbon!
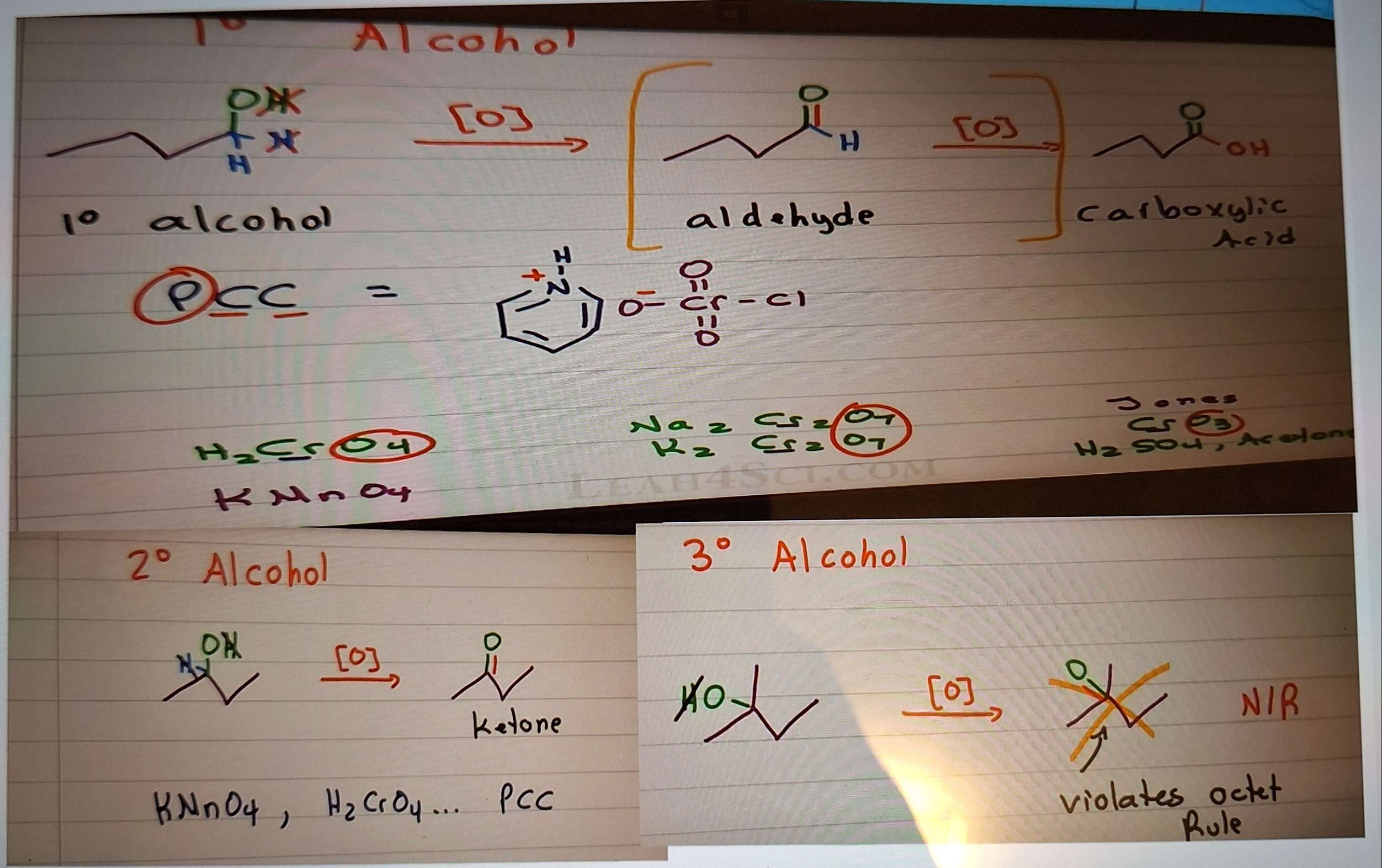
-
Swern Oxidation
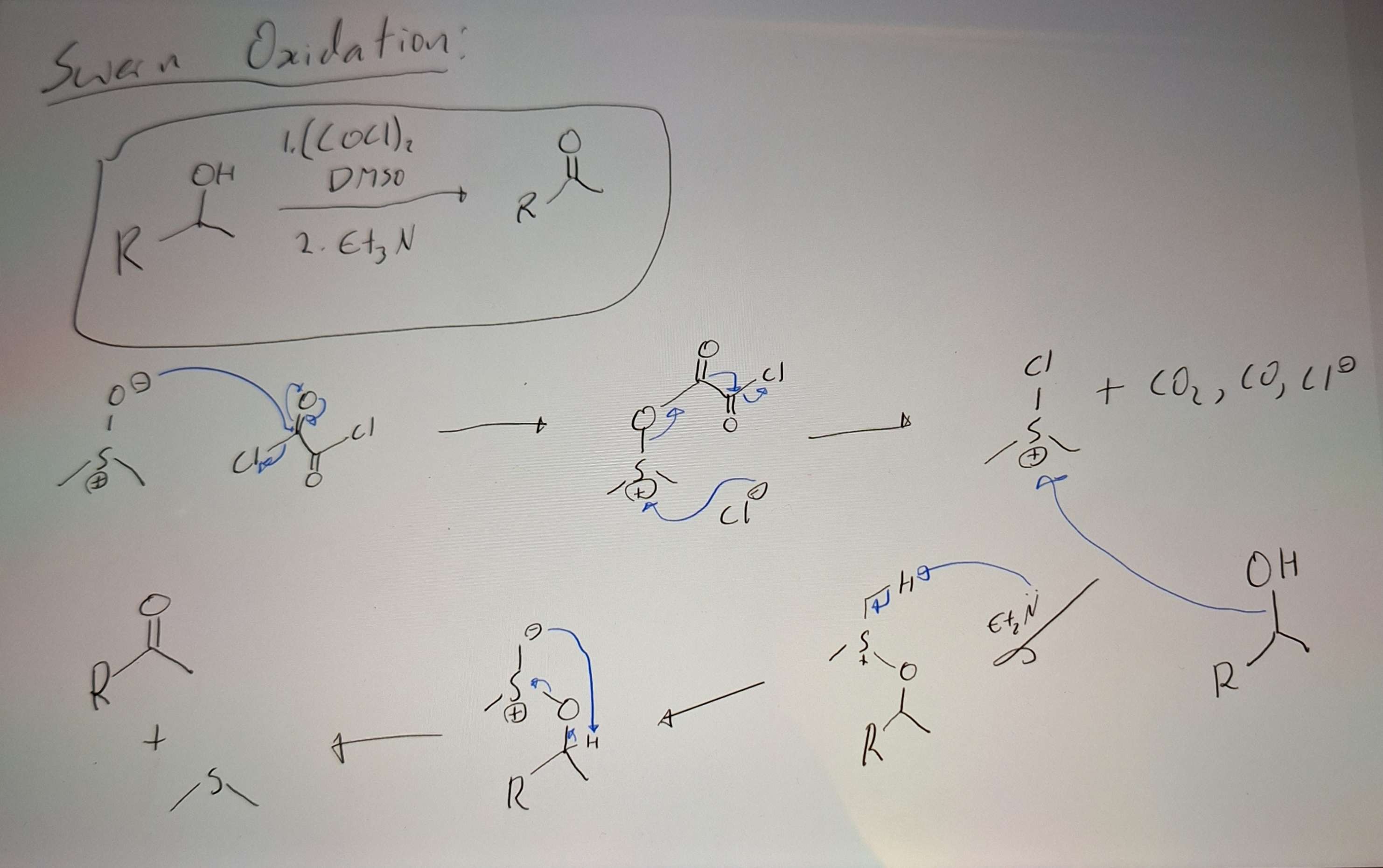
-
Reductions of Aldehydes and Ketones - How to make C=O ----> to C-OH
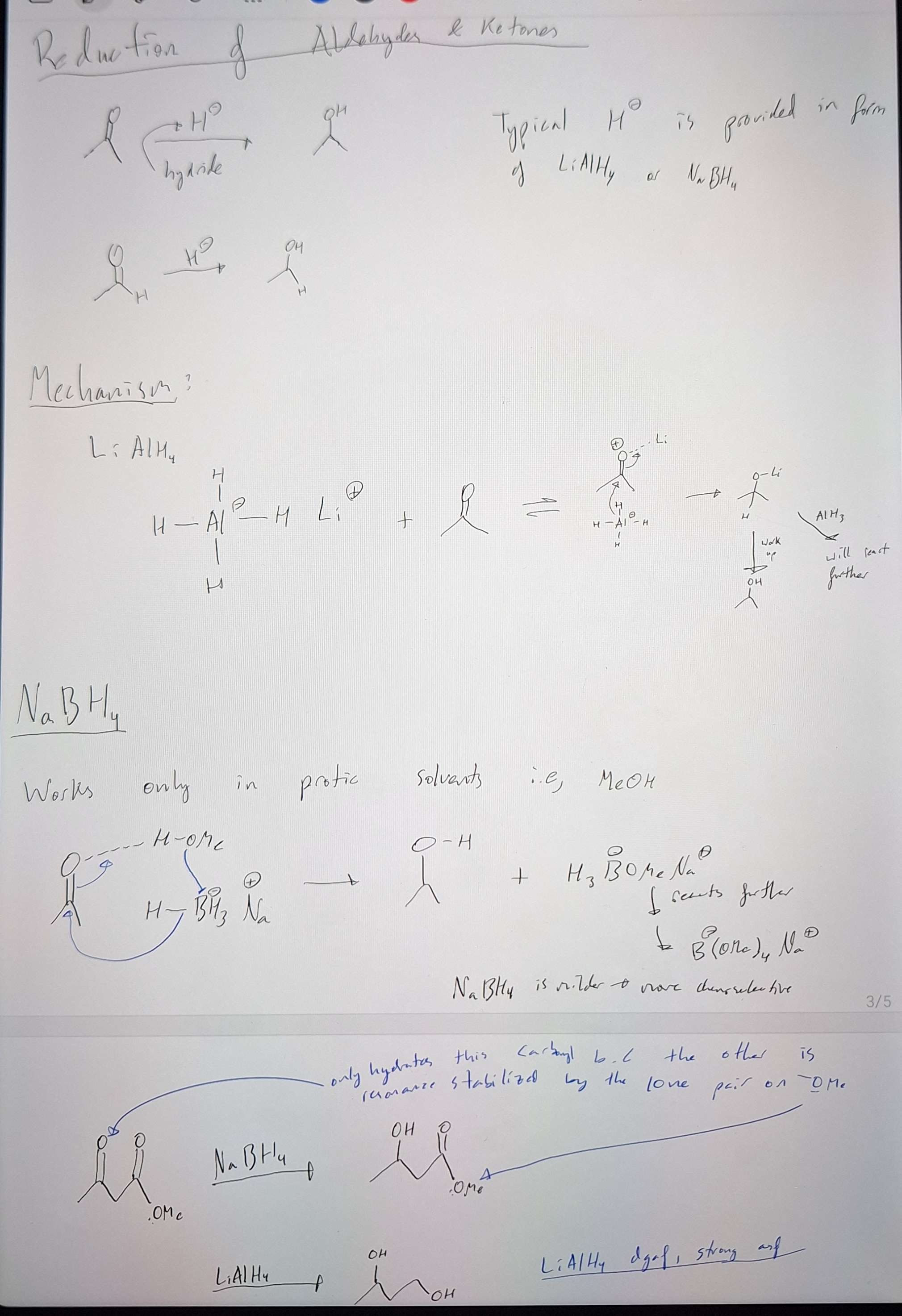
-
Des Martin Oxidation
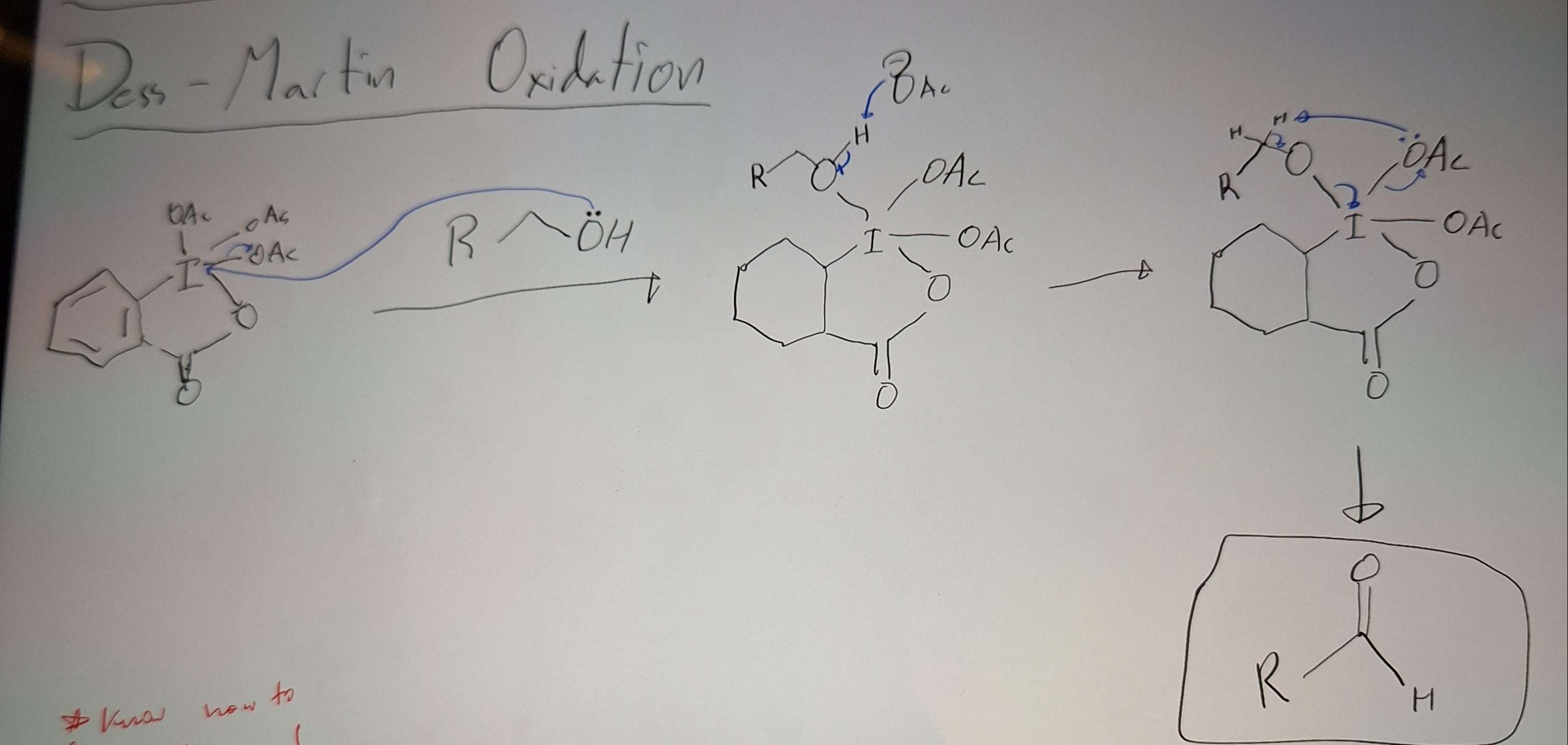
-
Jones Oxidation Mech
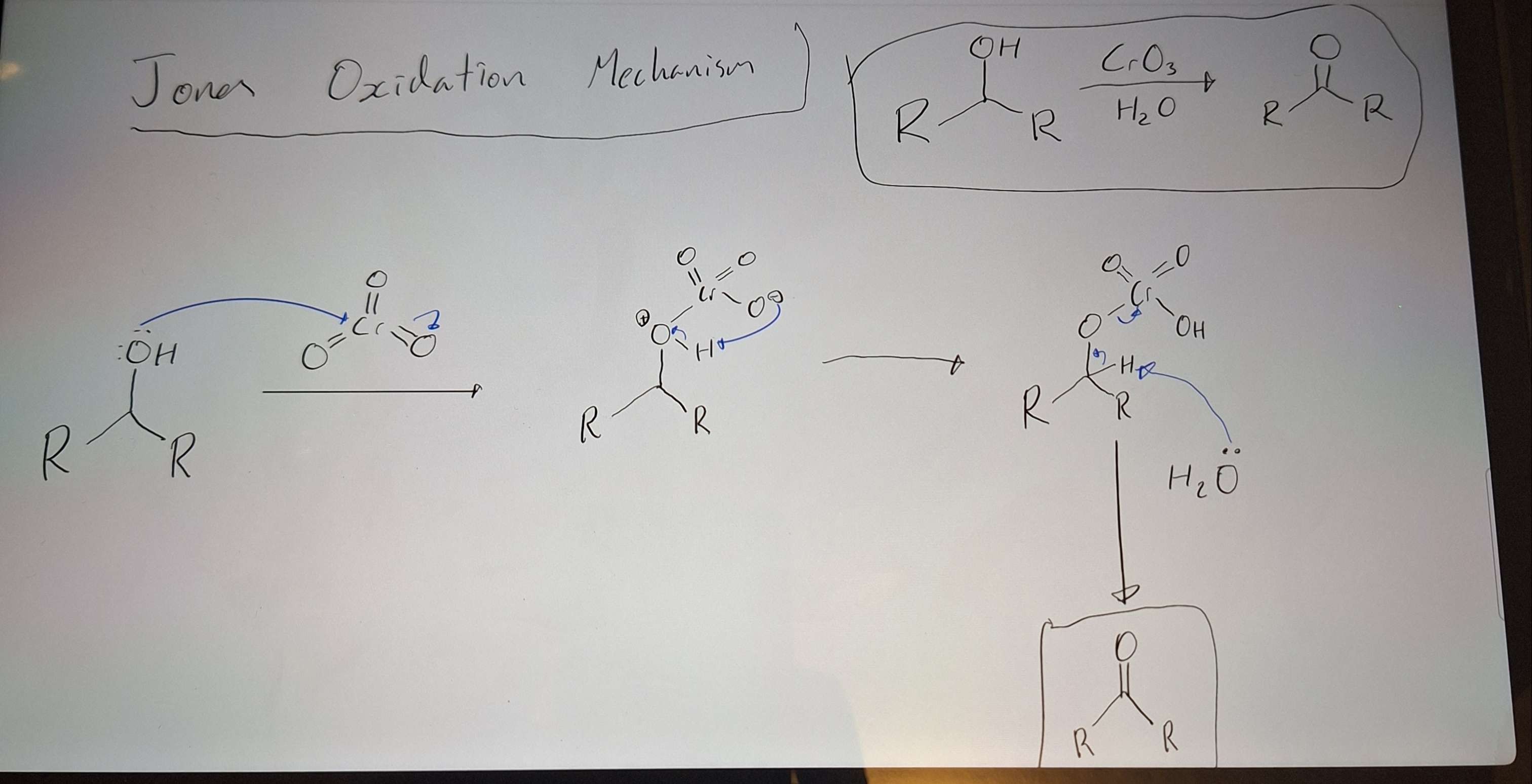
-
PCC oxidation mech
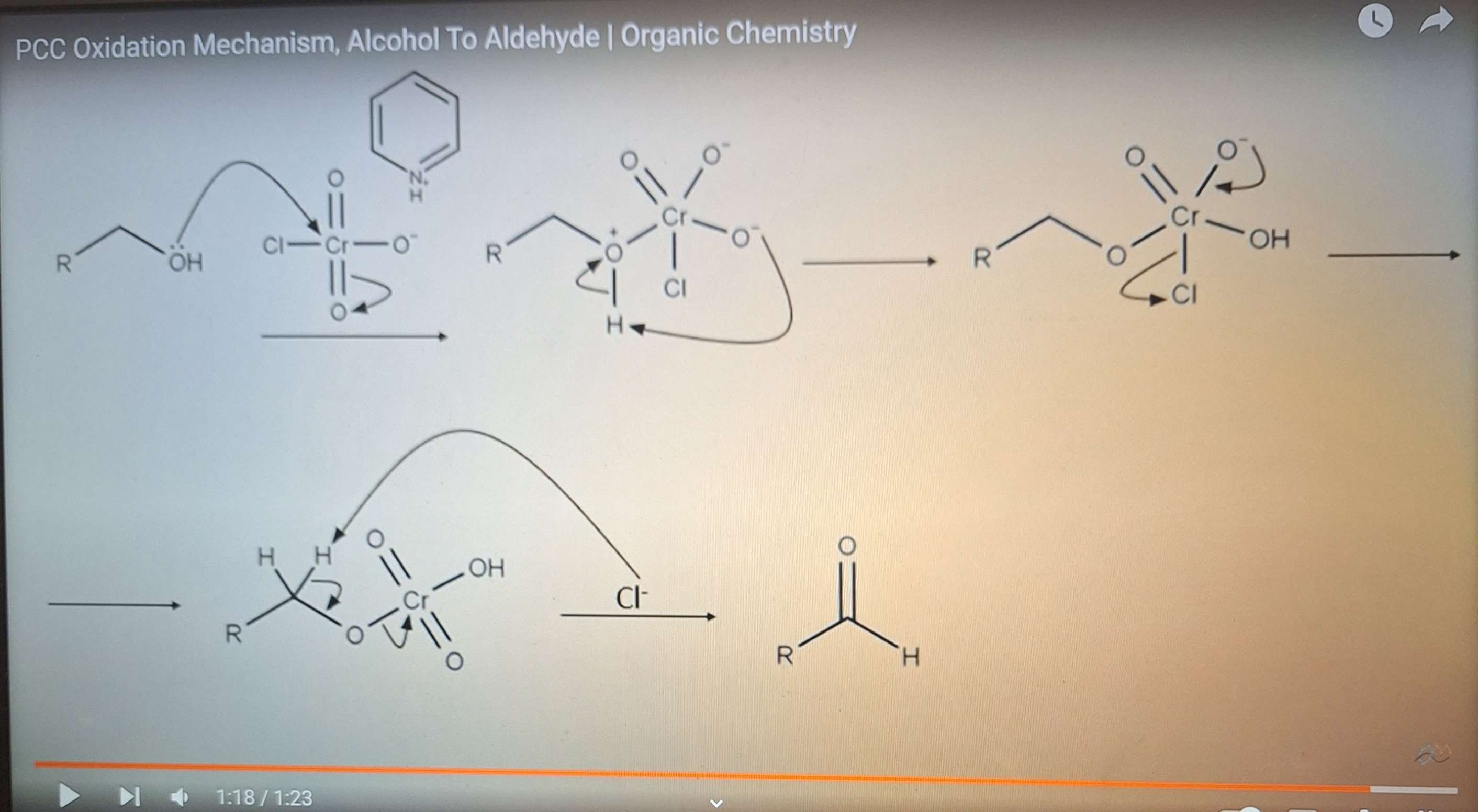
-
Friedel - Craft Acylation (installs carbonyl onto rings like benzene)
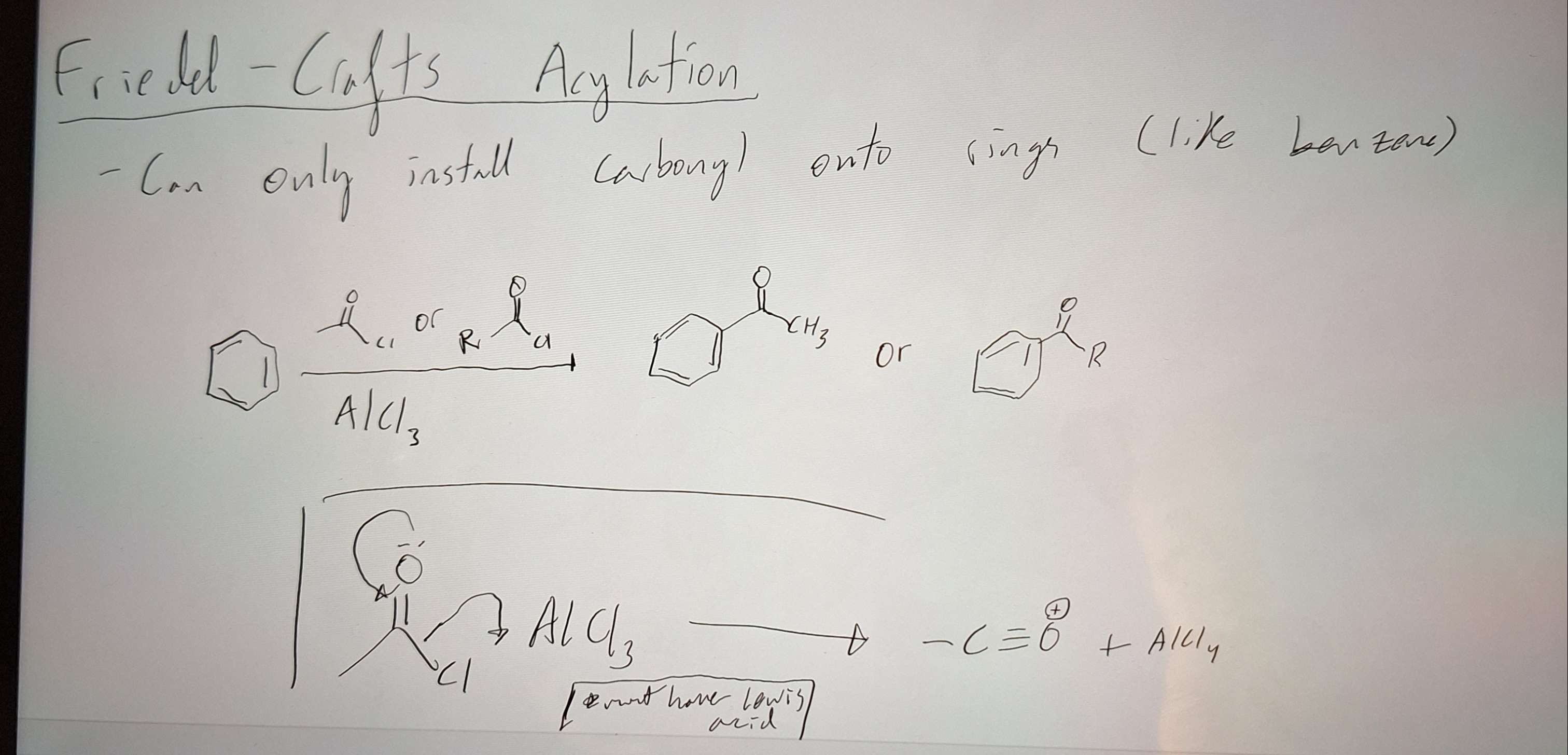
-
Hydration of Alkynes mech:
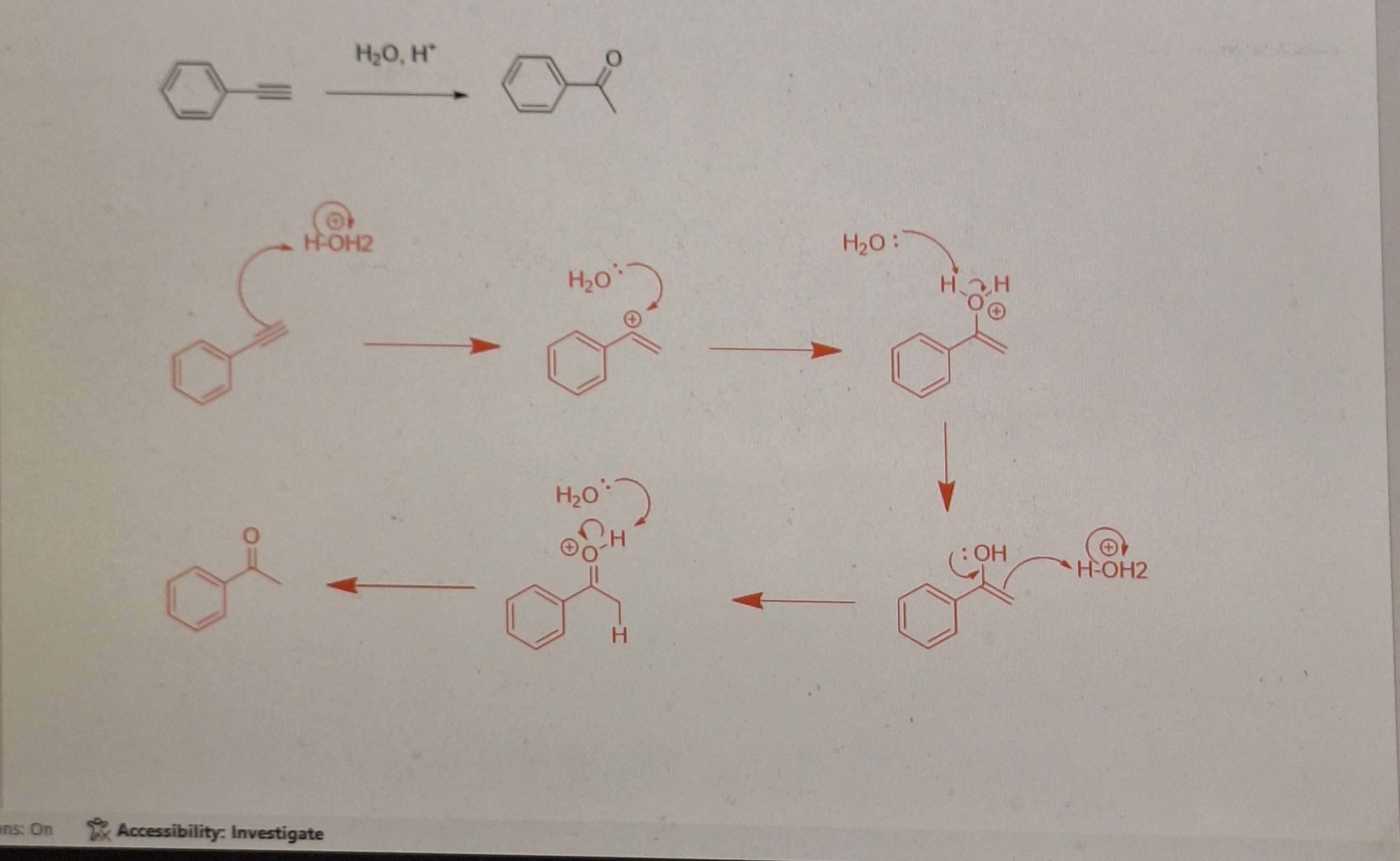
-
Hydration of Alkenes (Wacker Oxidation) - C=C to C=O
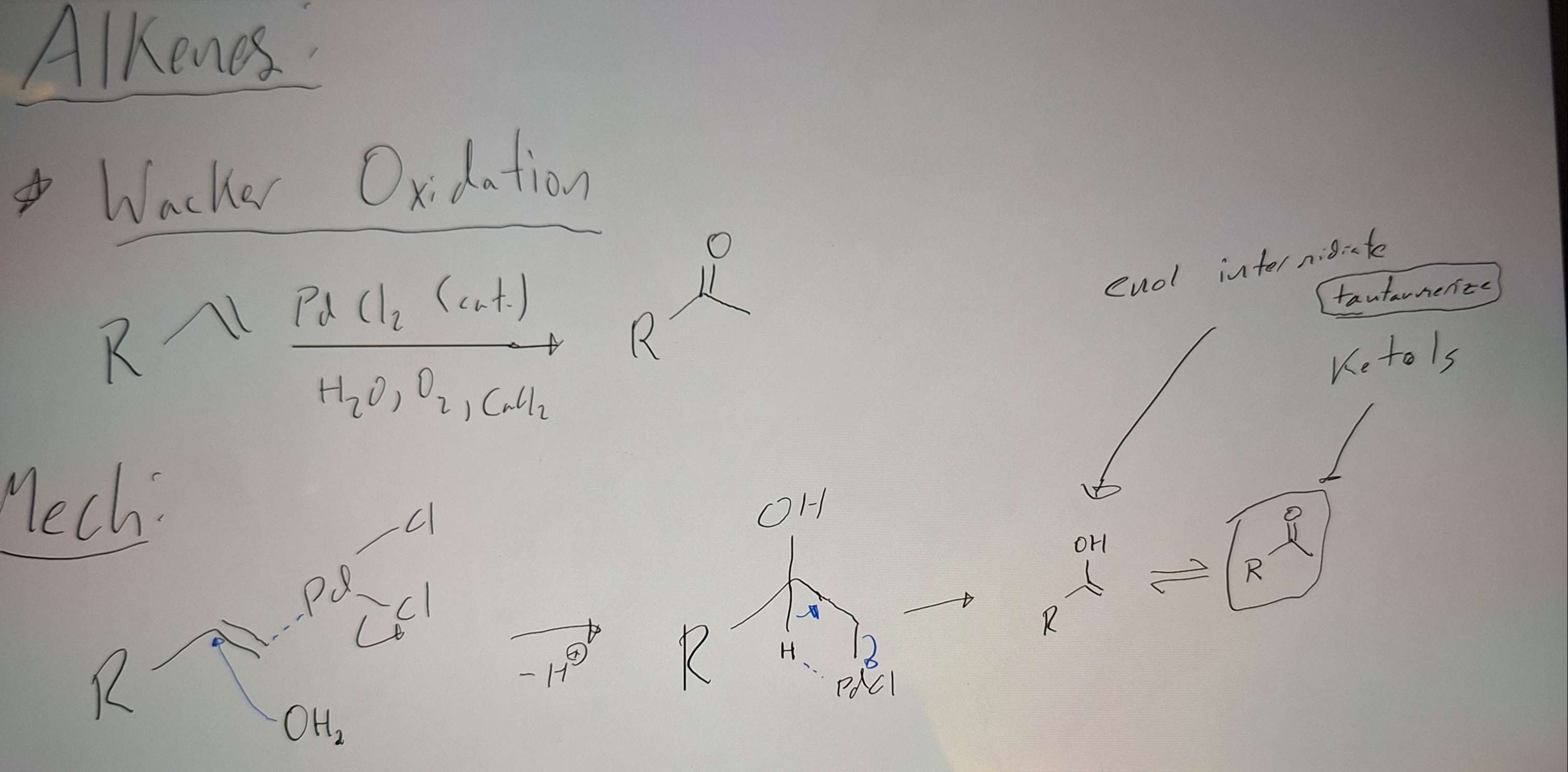
-
Hydroboration Oxidation
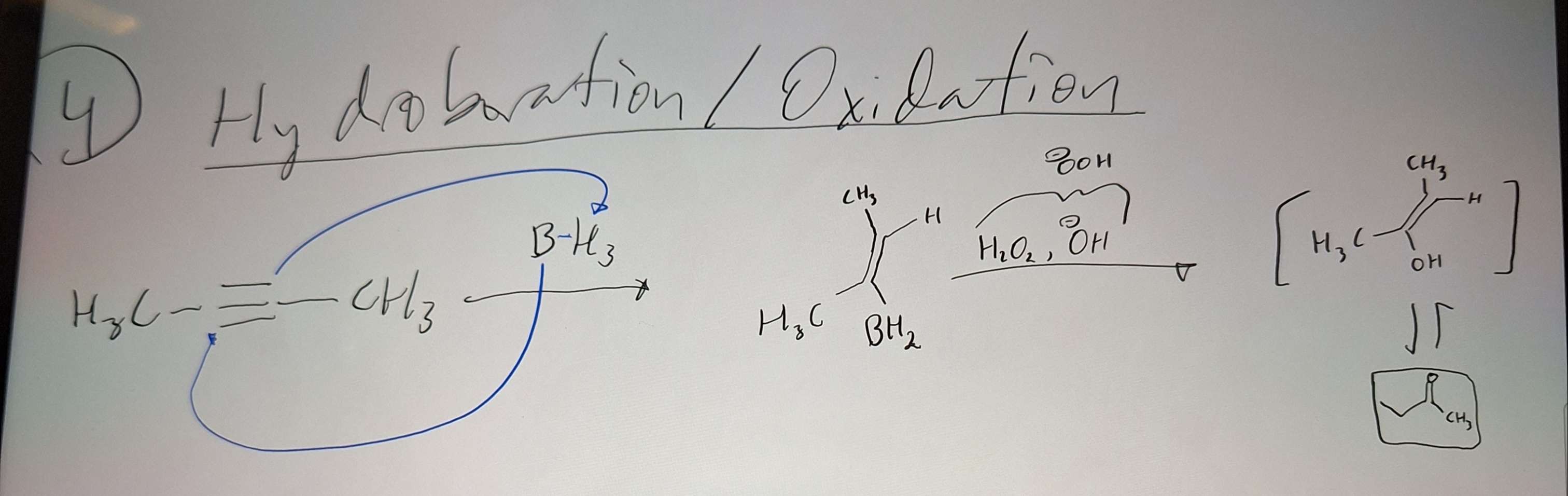
-
Ozonolysis of Alkenes
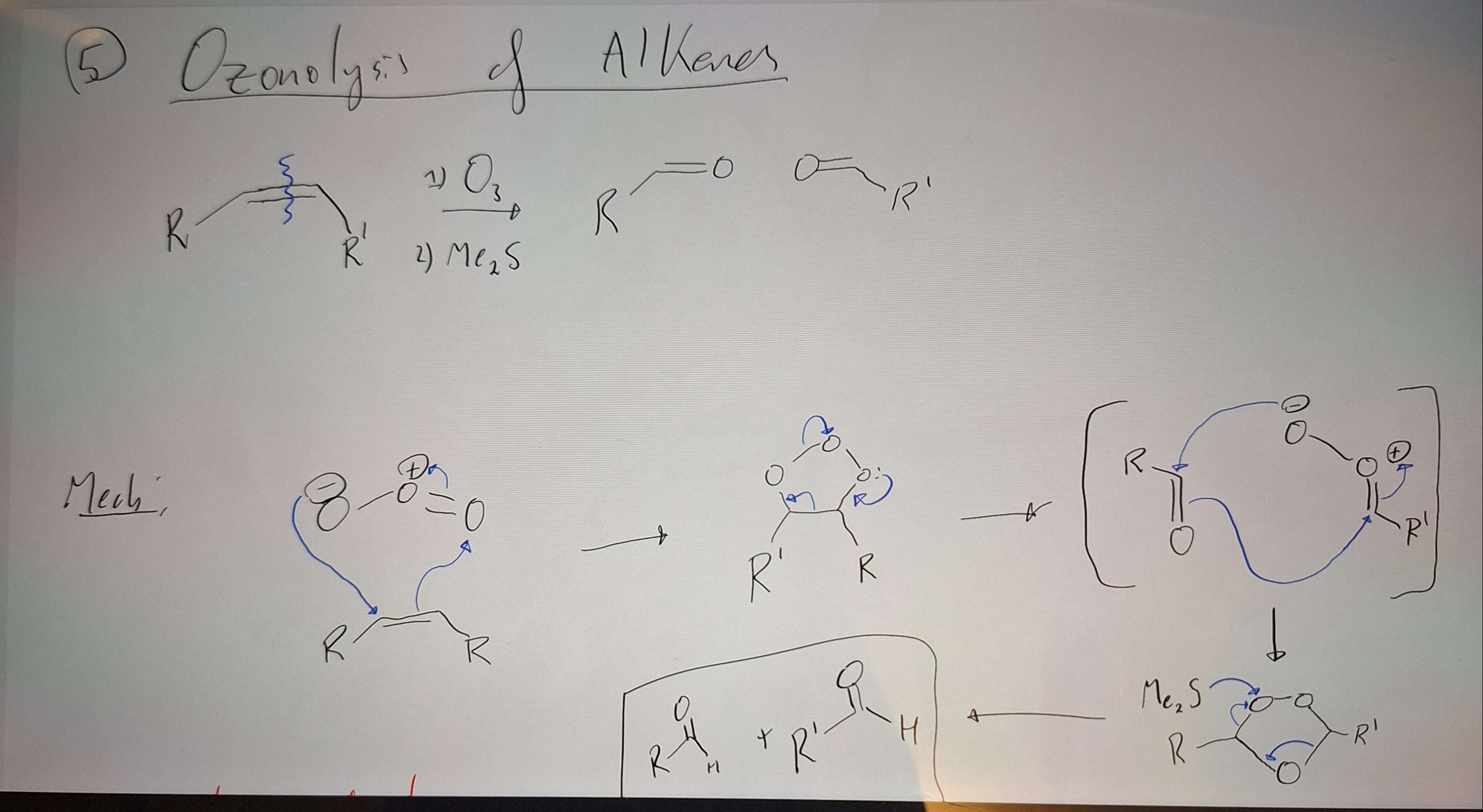
-
Reactions of Aldehydes and Ketones
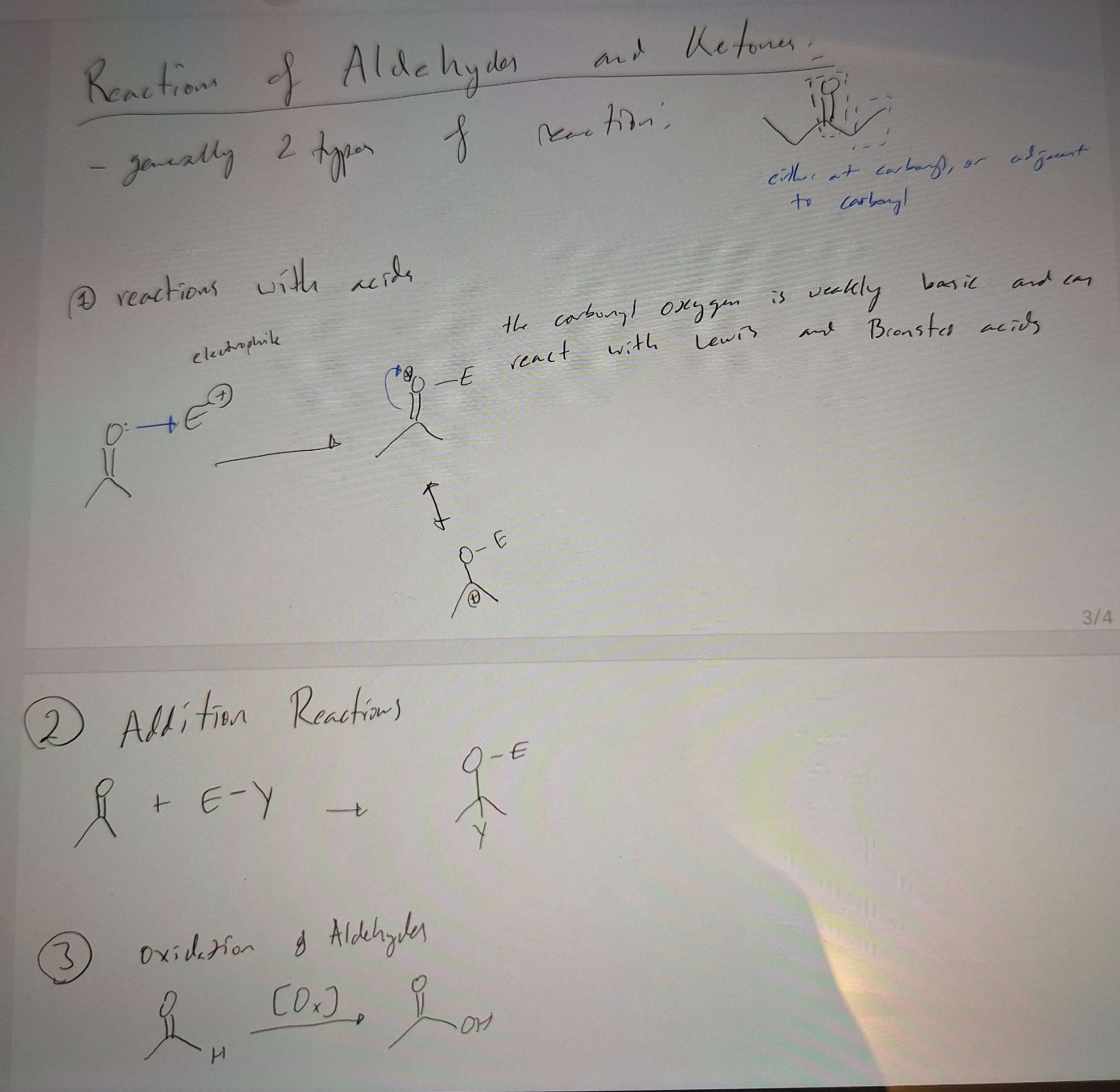
-
Pinacol reaction
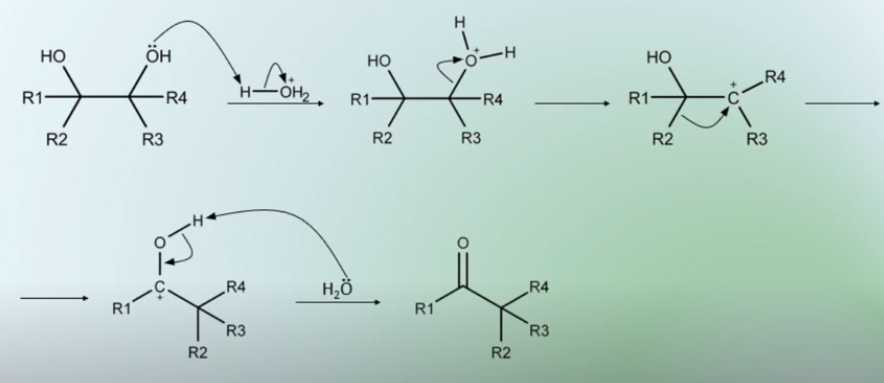
-
Reversible addition rxns for aldehydes and ketones + HCN and H2O
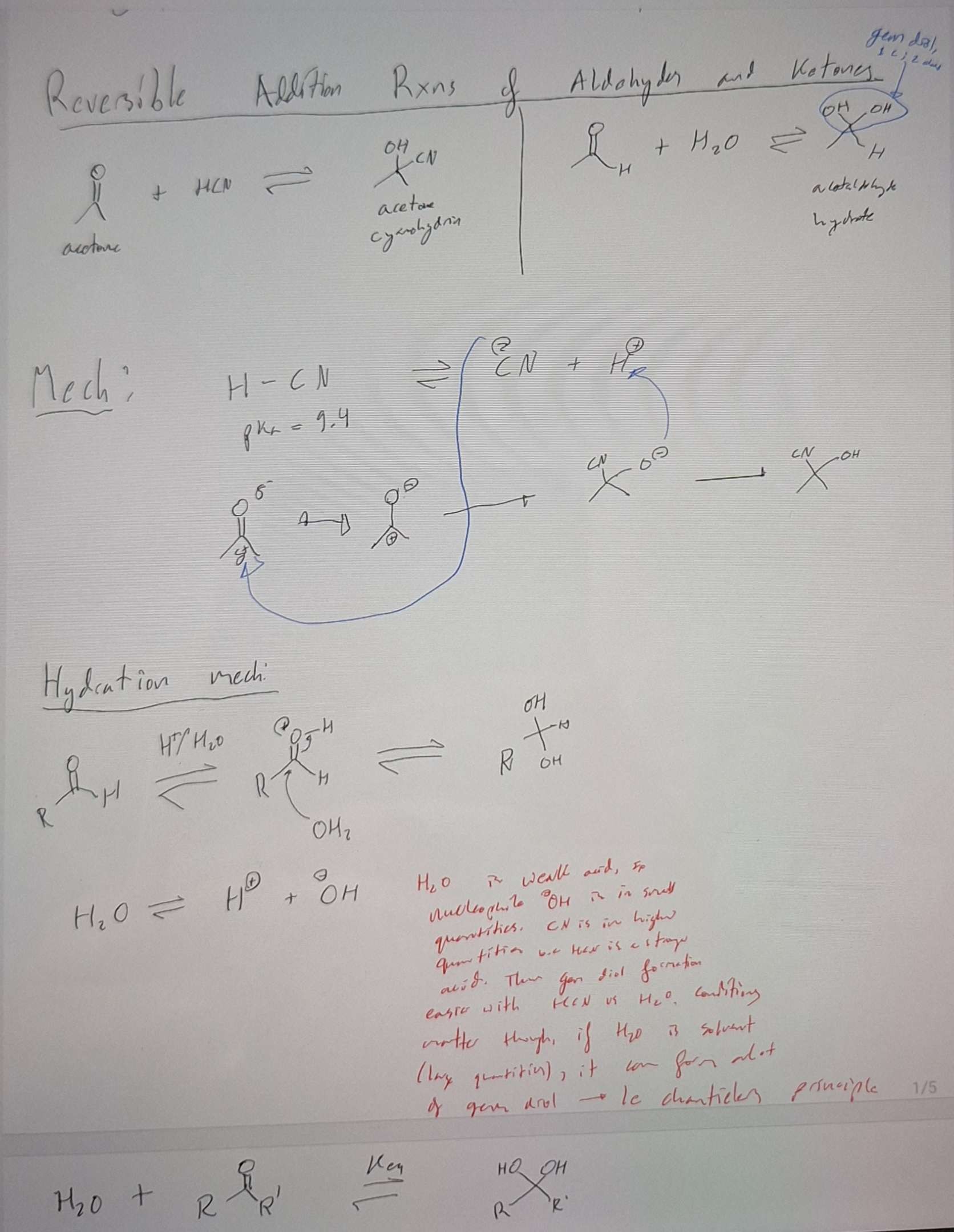
-
Aldehydes and Ketones reactivity with respect to steric and inductive (and resonance) effects of R groups

-
Grignard rnxs with aldehydes and ketones
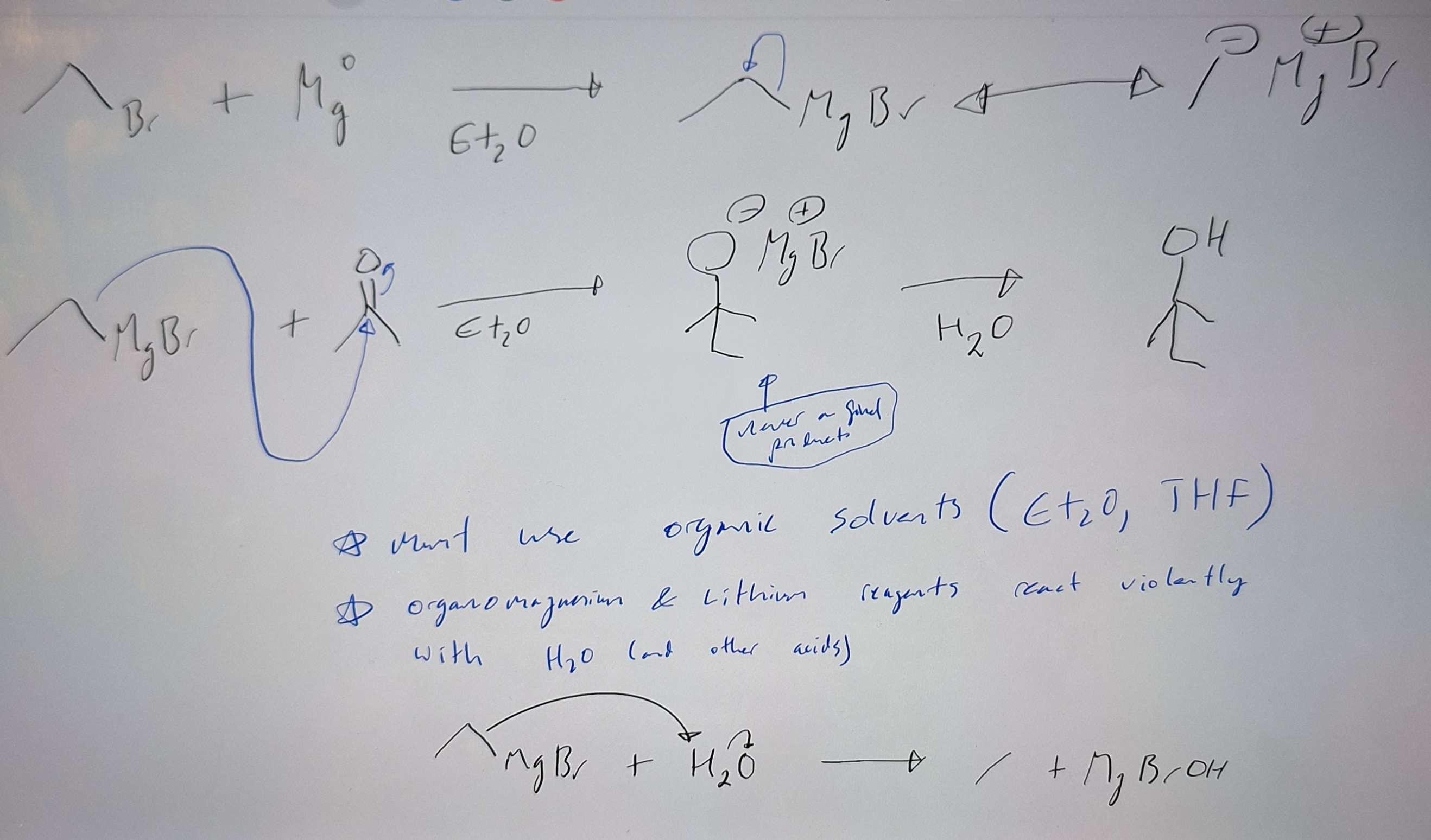
-
OrganoLithiums and OrganoSodiums to go from C=O to C-OH
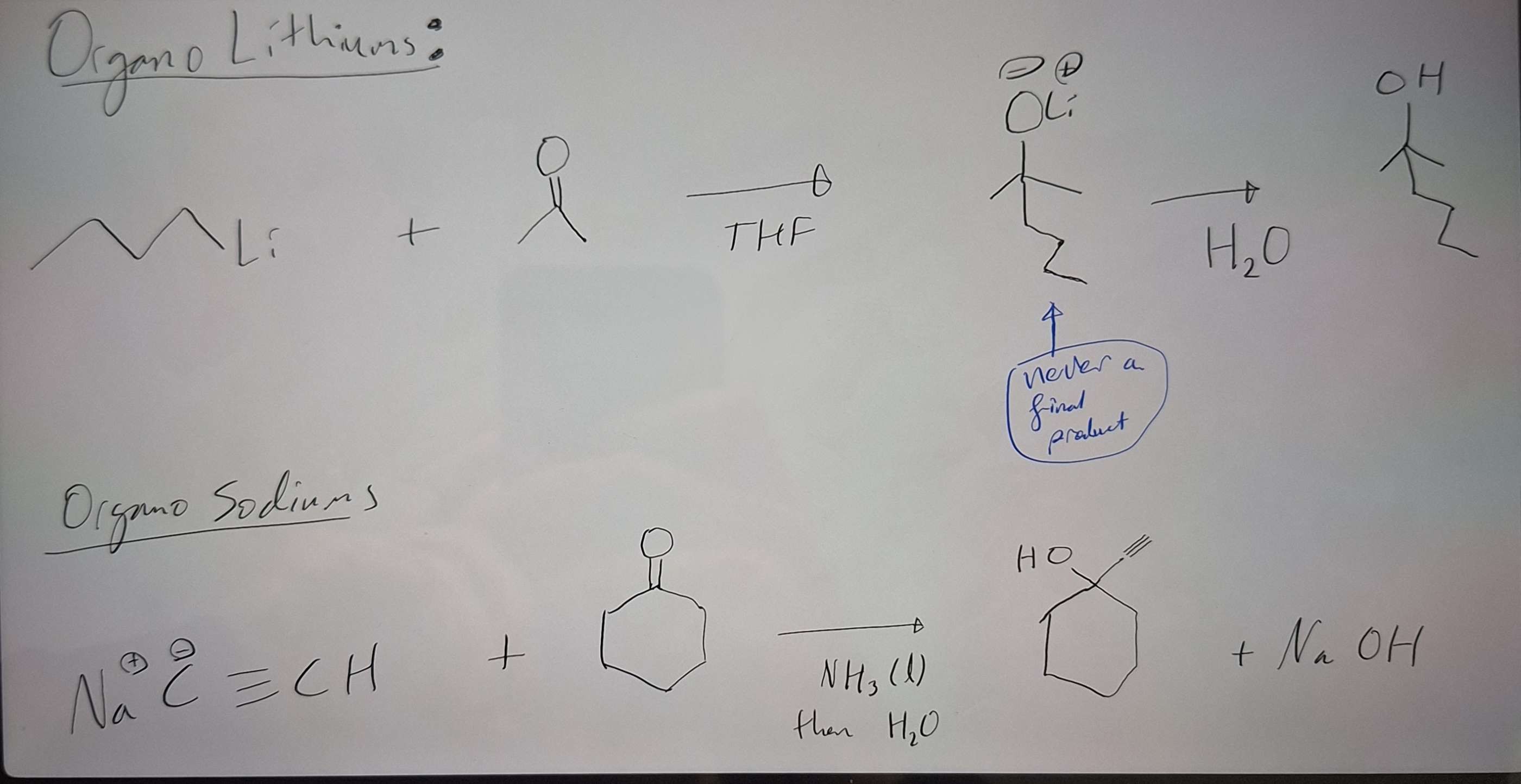
-
Preparation of Organometallic reagents