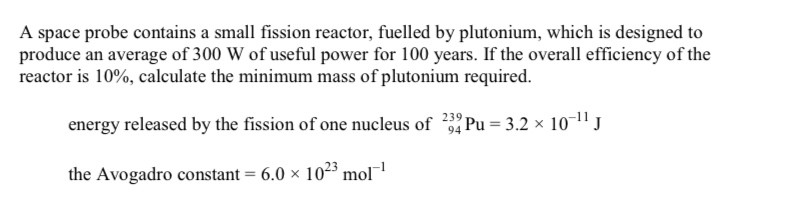-
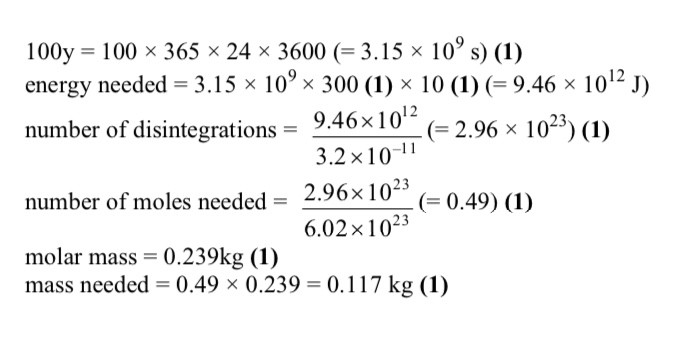
What element is X
Krypton
-
-
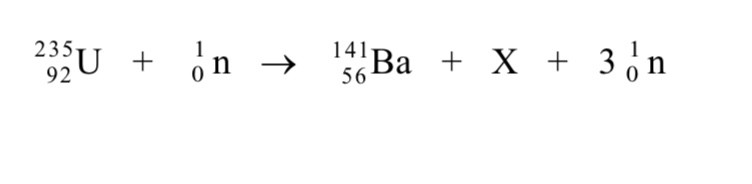
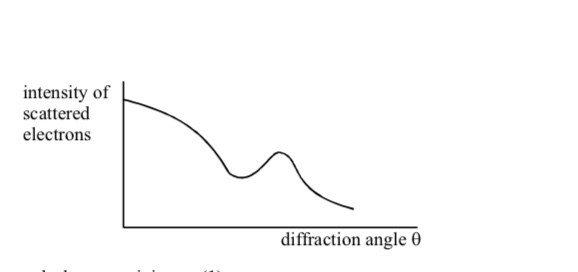
Why are high energy electrons used to determine nuclear size
Not subject to strong nuclear force
-
Electron diffraction experiments have been performed on a range of different nuclei to give information about nuclear density and average separation of particles in the nucleus
Nuclear density and average separation of nucleons are constant
-

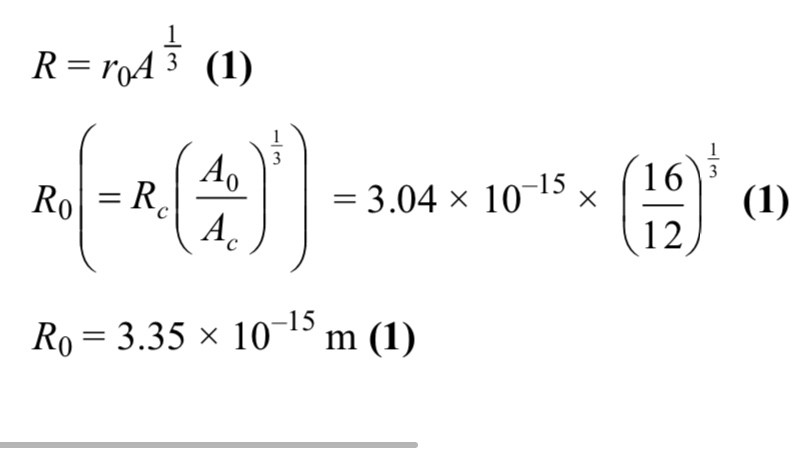
-
An argon atom formed by electron capture releases an x ray photon how ?
Orbital electron vacancy due to electron capture
Outer electron fills vacancy and emit x ray photon
-

Alpha
Two different track lengths
Short range particles have lower energy than long range particles
Particles in each range have same energy
-
Which of the two radioactive isotopes, plutonium –239 or the uranium isotope, has the longer half-life?
U-235
Inverse relationship Of half life and alpha particle energy
-

Half life - 50s
Decay constant - 0.014 s^-1
Number of particles initially - 1.7 x 10^7
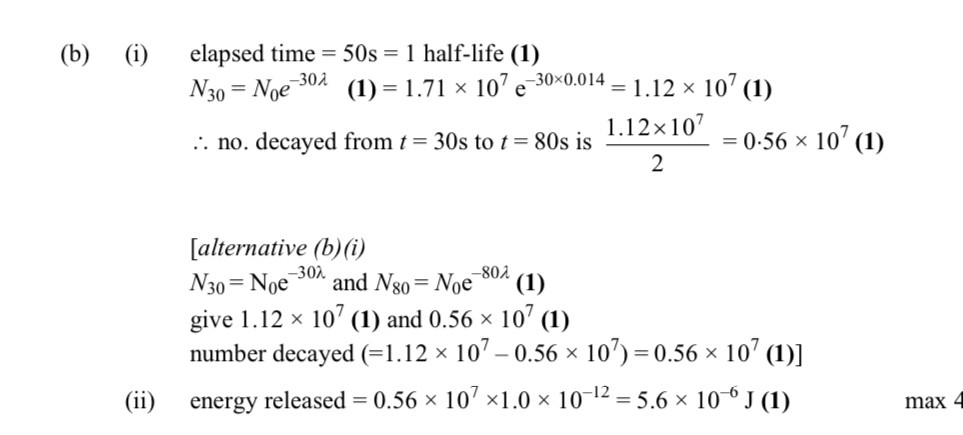
-
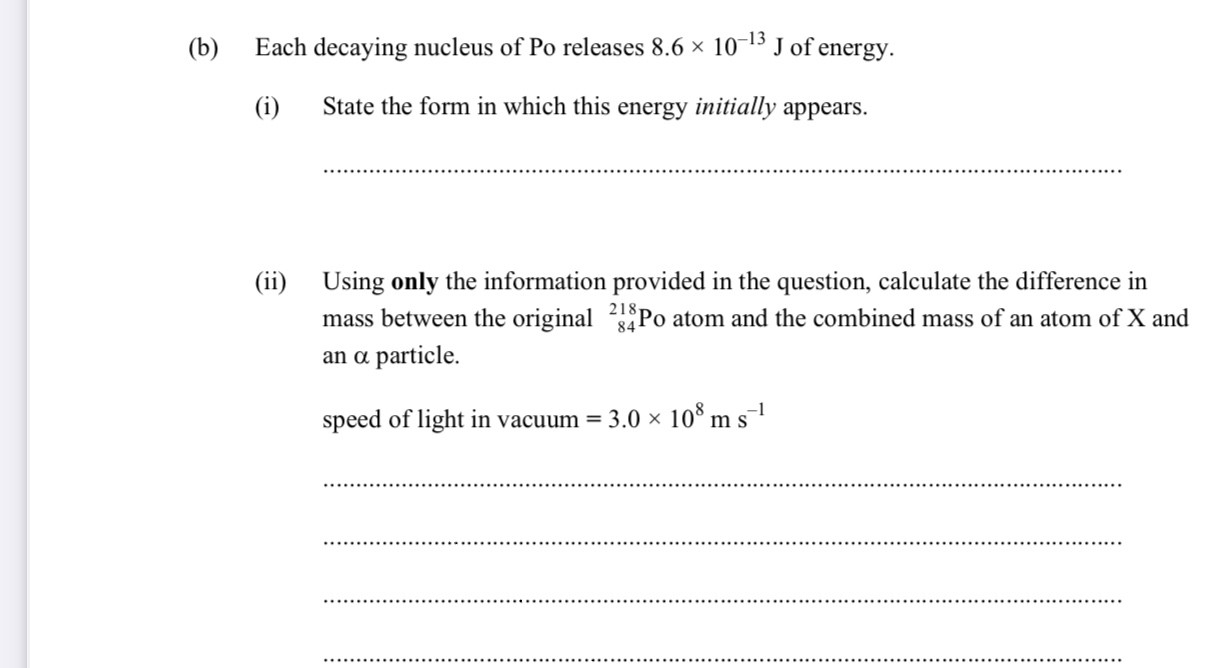
82 p 214 nucleon
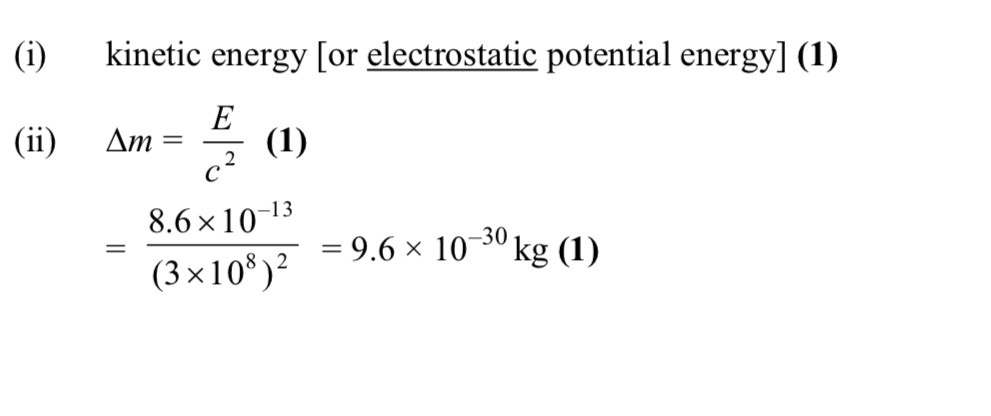
-