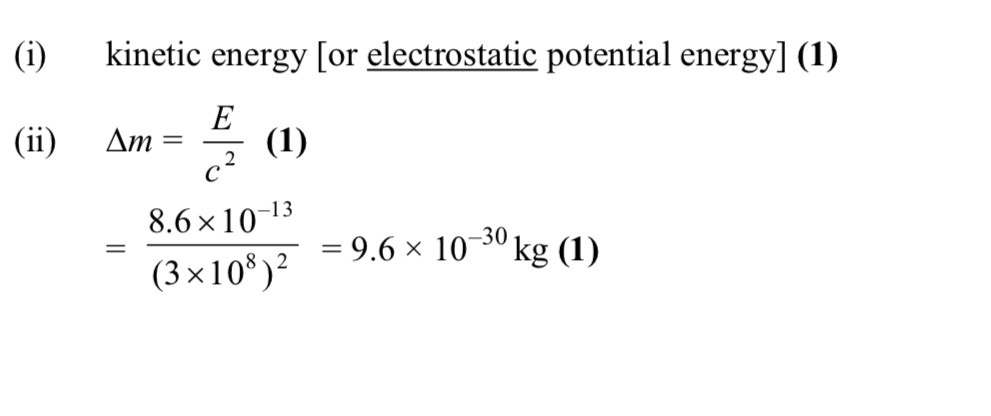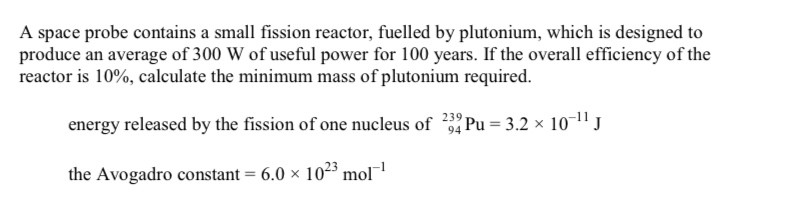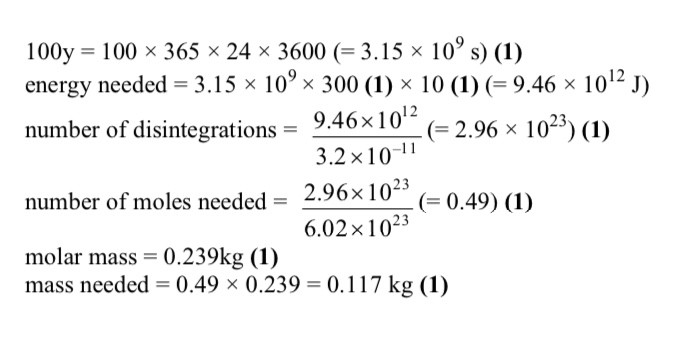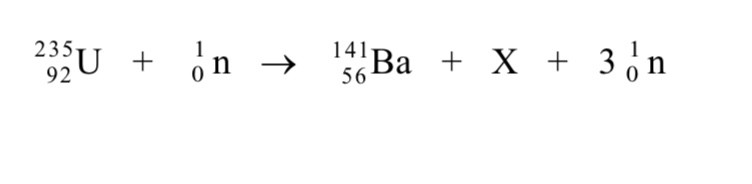
What element is X
Krypton
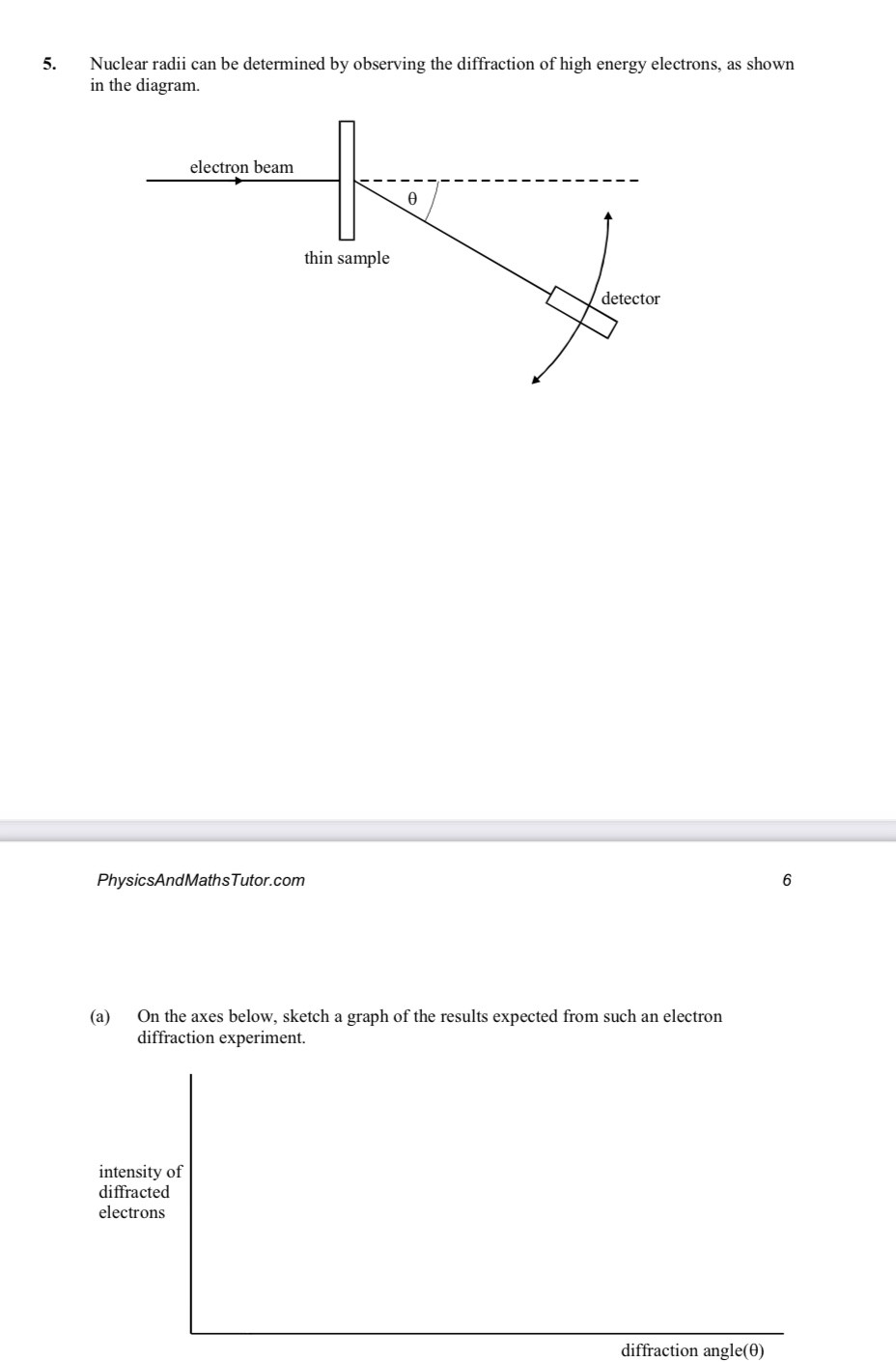
Why are high energy electrons used to determine nuclear size
Not subject to strong nuclear force
Electron diffraction experiments have been performed on a range of different nuclei to give information about nuclear density and average separation of particles in the nucleus
Nuclear density and average separation of nucleons are constant

An argon atom formed by electron capture releases an x ray photon how ?
Orbital electron vacancy due to electron capture
Outer electron fills vacancy and emit x ray photon

Alpha
Two different track lengths
Short range particles have lower energy than long range particles
Particles in each range have same energy
Which of the two radioactive isotopes, plutonium –239 or the uranium isotope, has the longer half-life?
U-235
Inverse relationship Of half life and alpha particle energy

Half life - 50s
Decay constant - 0.014 s^-1
Number of particles initially - 1.7 x 10^7
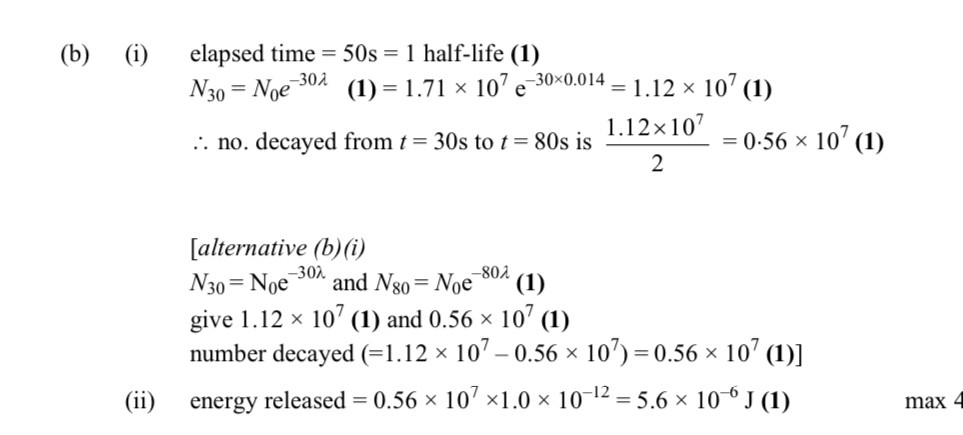
82 p 214 nucleon