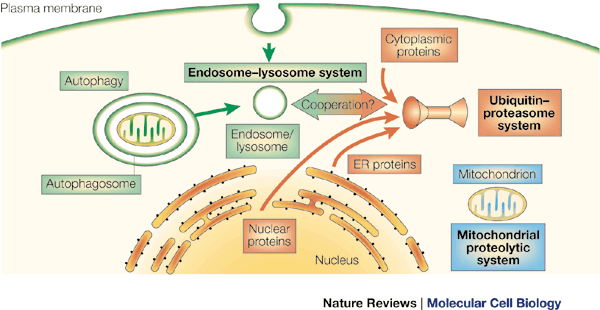
Intracellular proteolysis (The Living Cell) UNFINISHED
Proteolysis refers to the cleavage of proteins or peptides through hydrolysis of peptide bonds. These reactions are catalyzed by a large family of enzymes called proteases. Proteases are classified into different groups according to their reaction mechanism and the nature of key amino acids at the enzymes' active site. Other terms used to describe proteases are based on whether the enzyme recognizes sites internal to the substrate protein (endopeptidases) or whether residues are removed from the termini (exopeptidases). Proteases that cleave peptide bonds only in the context of very specific sequences generally serve to convert a substrate protein from an inactive to an active form; examples discussed are the ER signal peptidase, the processing of many prohormones in the Golgi and the activation of certain digestive enzymes of the small intestine. The role of proteases with low substrate specificity is the complete degradation of proteins down to very short peptides or amino acids. Inside cells such enzymes must be compartmentalised in order for degradation to be controlled. The cellular compartments where degradative proteolysis takes place are the acidic lumen of lysosomes and barrel-shaped protein structures called proteasomes. Marking proteins for degradation by the proteasome involves the attachmend of a chain of ubiquitin molecules in a process catalysed by enzymes called E1, E2 and E3.
-
What are Cathepsins?
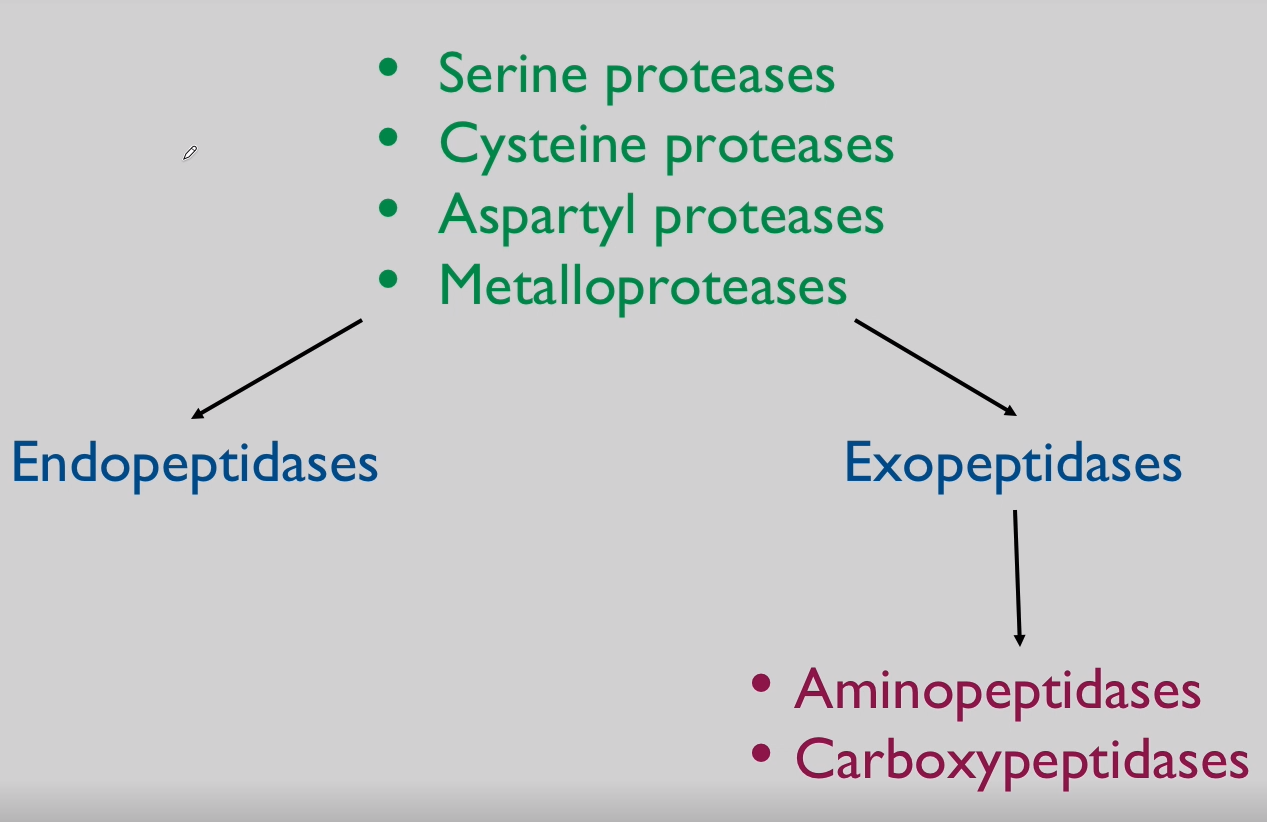
Cathepsins:
Family of lysosomal proteases.
Includes cathepsin B, L, D, and others.
Proteases = Proteinases = Peptidases = Cathepsins
-
Specific sequences in proteolysis allow for?
-Protein Activation
-
Non-specific sequences in proteolysis allow for?
-Protein degradation
-
Zymogen, proprotein or proenzymes are terms often used to describe protein on It's what state?
Inactive
-
What is the role of Chymotrypsinogen? (3)
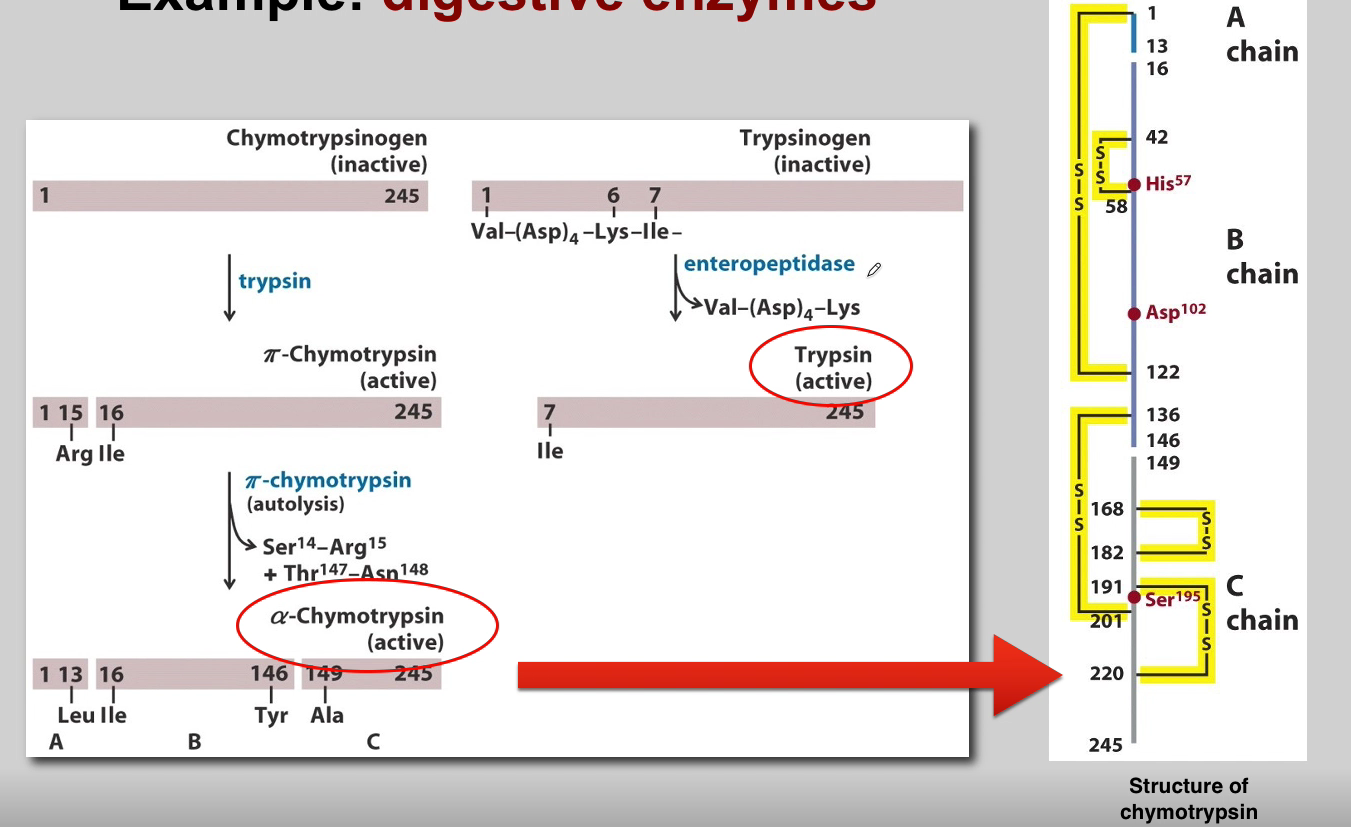
Chymotrypsinogen:
Proenzyme Form: Chymotrypsinogen is the inactive proenzyme (zymogen) of chymotrypsin.
Secreted by Pancreas: Released by the pancreas into the small intestine.
Activation: Converted into active chymotrypsin by proteolytic cleavage.
-
What is the role of Trypsin? (3)
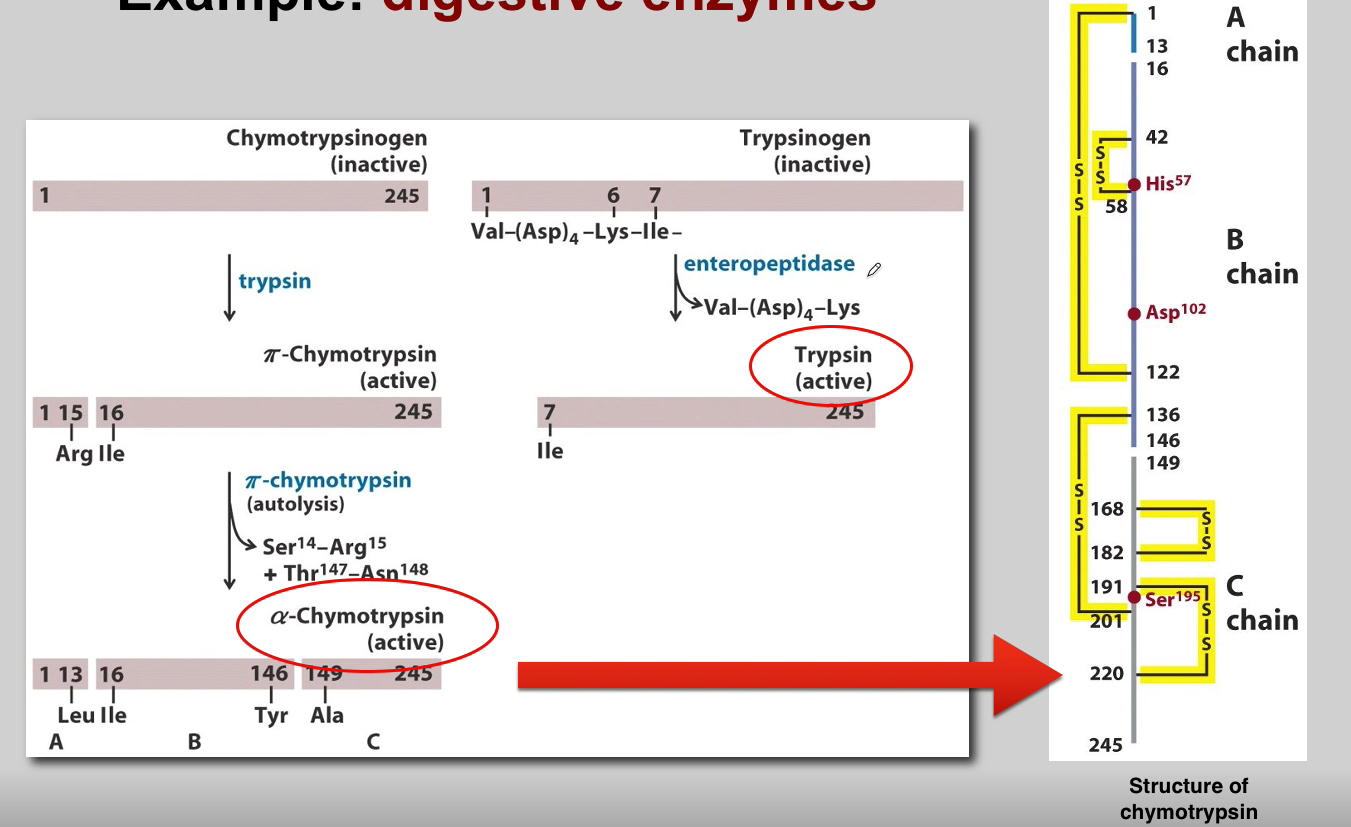
Trypsin:
Activation of Other Zymogens: Acts as an initiator of a cascade of zymogen activations.
Activation of Chymotrypsinogen: Activates chymotrypsinogen to chymotrypsin.
Autocatalysis: Activated trypsin can activate other trypsinogen molecules through autocatalysis.
-
What is the role of Chymotrypsin? (3)
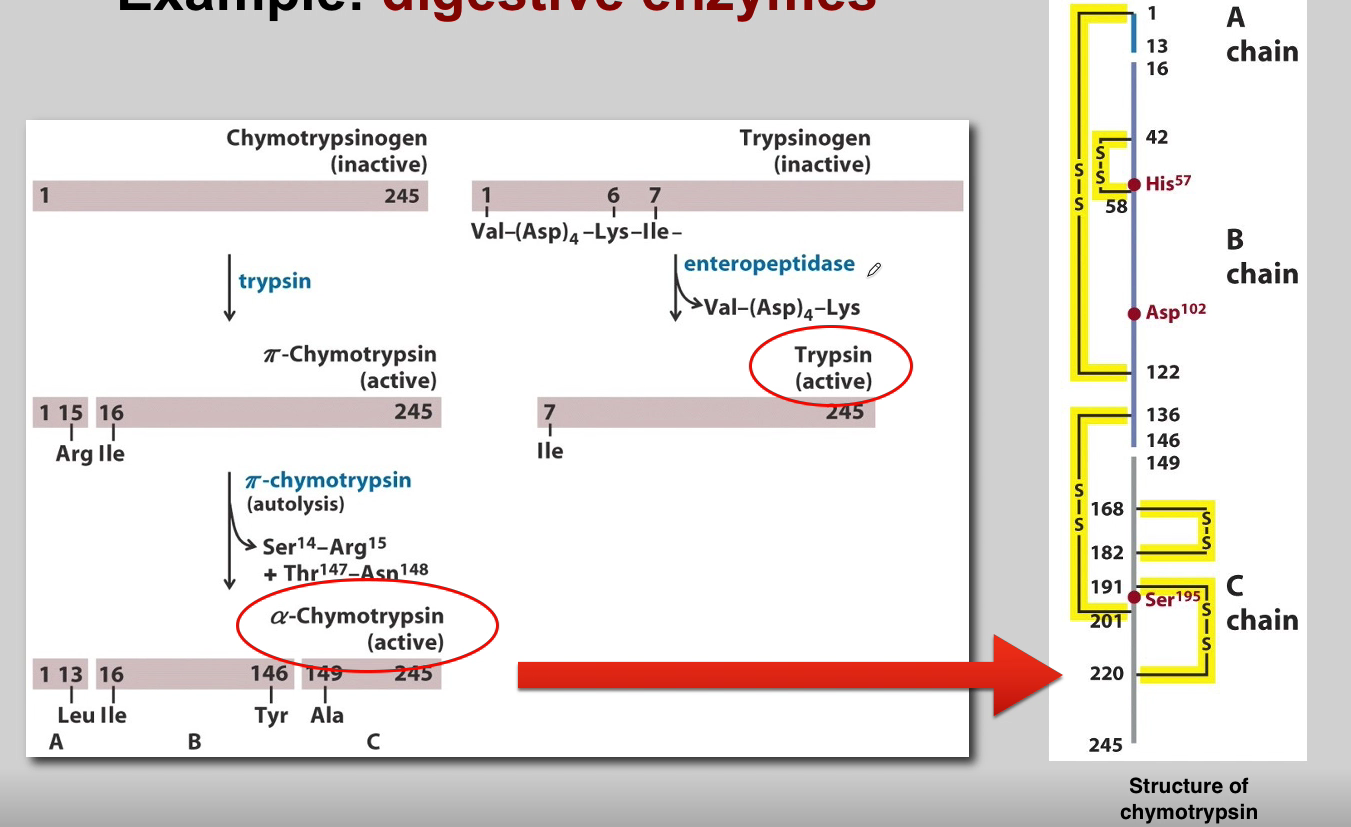
Chymotrypsin:
Proteolytic Activity: Active chymotrypsin is a protease that cleaves peptide bonds.
Substrate Specificity: Targets peptide bonds adjacent to aromatic amino acids.
Role in Digestion: Contributes to the digestion of proteins in the small intestine.
-
What is the role of Enteropeptidases? (3)
Enteropeptidases (Enterokinase):
Location: Located in the brush border of the small intestine.
Activation of Trypsinogen: Activates trypsinogen to trypsin.
Cascading Effect: Initiates the activation cascade of pancreatic zymogens.
-
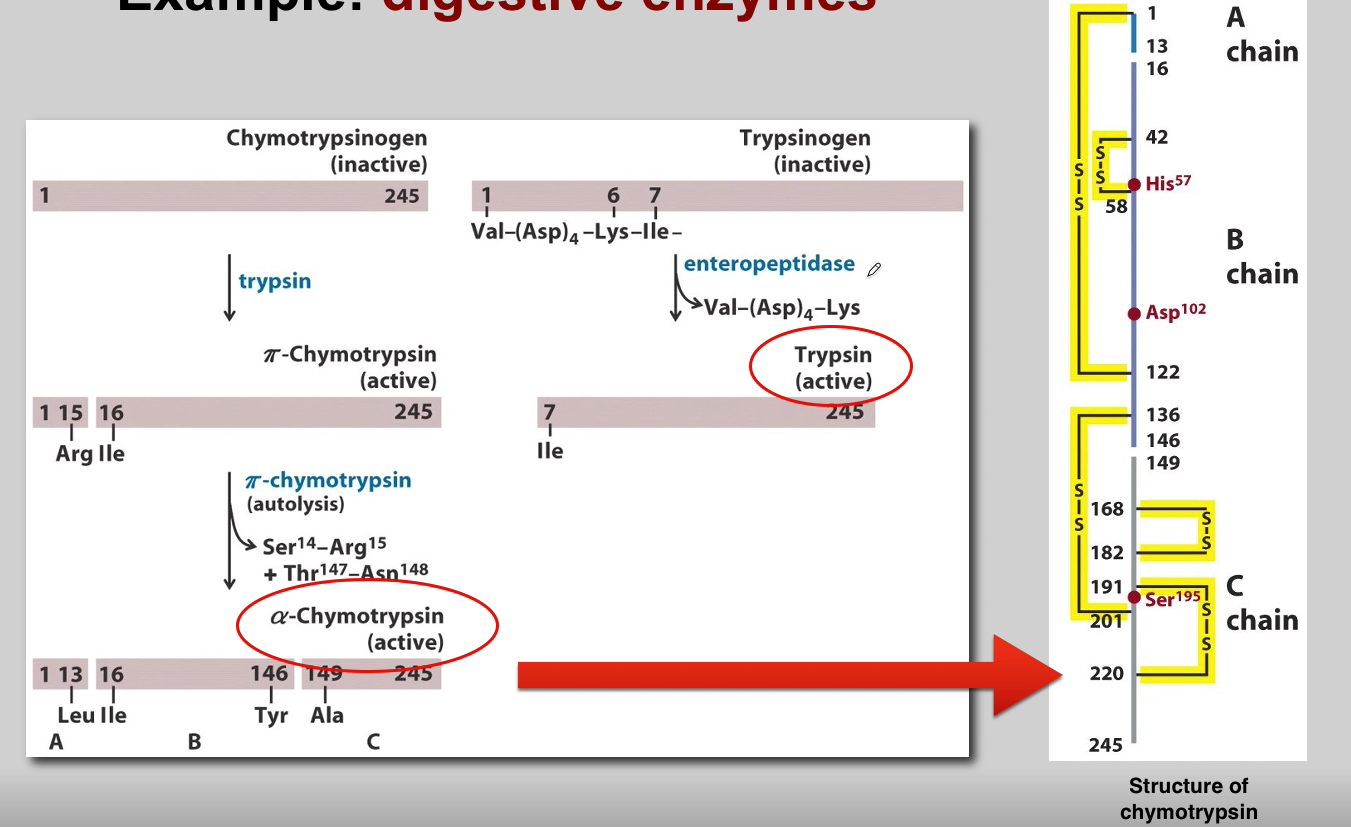
Picture outlining how Proteolytic processing of proopiomelanocortin in the Golgi gives rise to multiple peptide hormones
-
Insulin is a hormone that must also be processed by?
Proteolysis
-
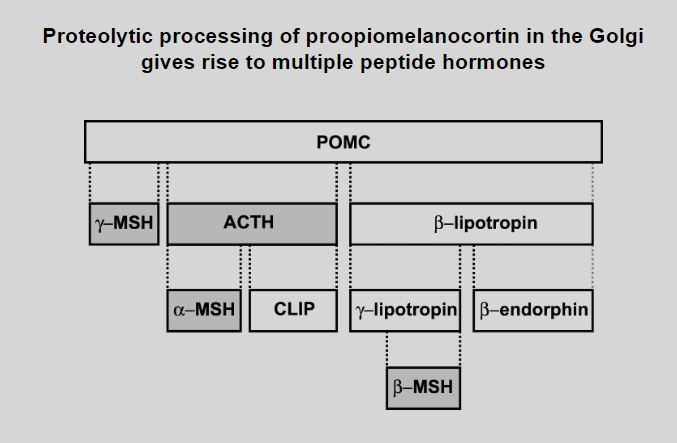
Outline the process how proteolysis prepares insulin, from preproinsulin to mature insulin
Preproinsulin Synthesis:
Synthesized in the endoplasmic reticulum (ER).
Proinsulin Formation:
Signal peptide removal creates proinsulin with A, B chains, and C peptide.
Transport to Golgi:
Proinsulin transported to the Golgi apparatus.
Proteolytic Cleavage:
Endopeptidases in secretory vesicles cleave C peptide from proinsulin.
Insulin and C Peptide:
Cleavage yields mature insulin and C peptide.
Storage and Release:
Stored in vesicles until stimuli trigger release.
Biological Activity:
Released insulin enters bloodstream, exerting biological effects.
C Peptide Function:
C peptide, though cleaved, has physiological functions.
-
What is another process that is caused by protein activation by proteolysis?
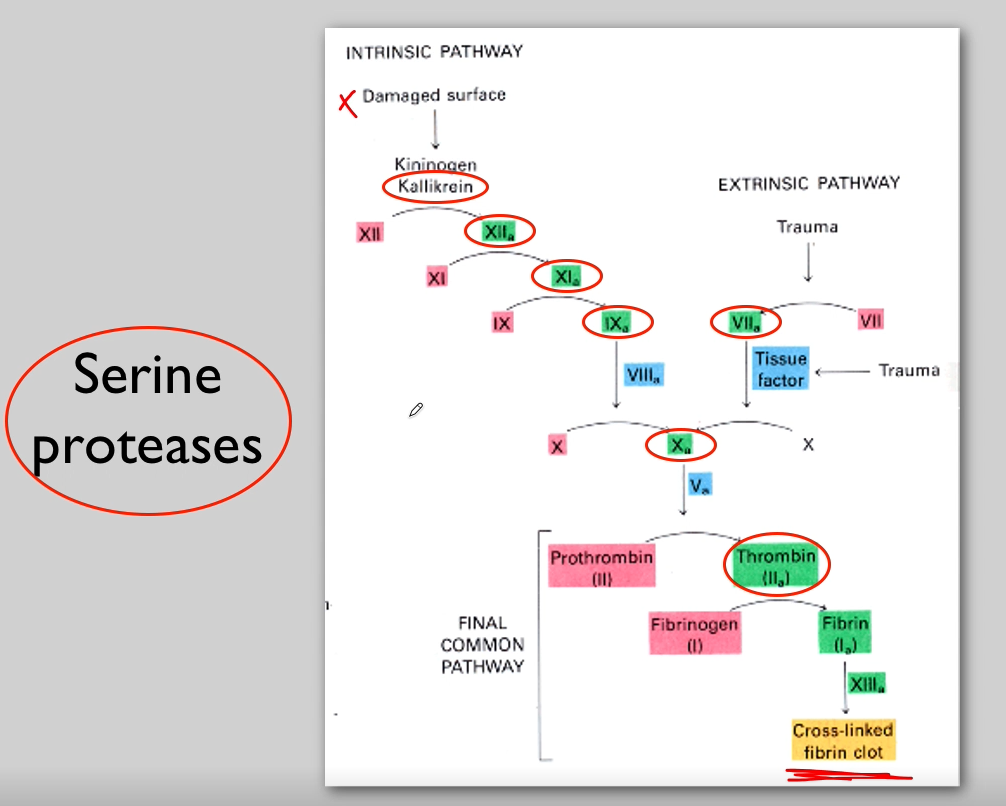
Clotting factors (By serine proteases)
-
Deficiencies of factor VIII or IX are the cause of what?
X-Linked haemophilia
-
What is X-linked haemophilia?
Inheritance: X-linked recessive genetic disorder.
Genes Involved: Haemophilia A (Factor VIII deficiency) or Haemophilia B (Factor IX deficiency).
Chromosomal Link: Mutations occur on the X chromosome.
Prevalence: Mainly affects males; females are carriers.
Bleeding Disorder: Impaired blood clotting due to deficient or dysfunctional clotting factors.
-
Name a famous cysteine protease
-Bromelain (found in pineapples)
-Papain (found in raw papaya fruit)

