What are Cathepsins?
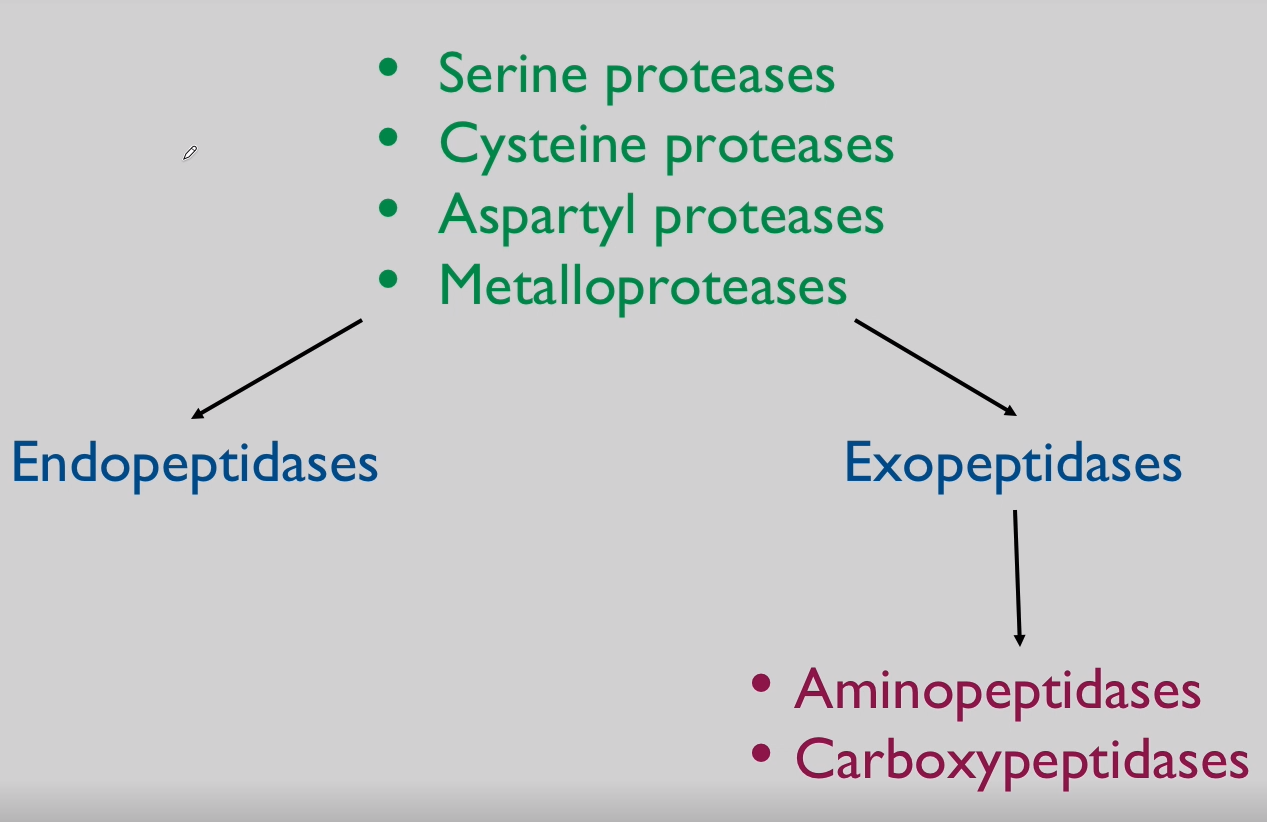
Cathepsins:
Family of lysosomal proteases.
Includes cathepsin B, L, D, and others.
Proteases = Proteinases = Peptidases = Cathepsins
Specific sequences in proteolysis allow for?
-Protein Activation
Non-specific sequences in proteolysis allow for?
-Protein degradation
Zymogen, proprotein or proenzymes are terms often used to describe protein on It's what state?
Inactive
What is the role of Chymotrypsinogen? (3)
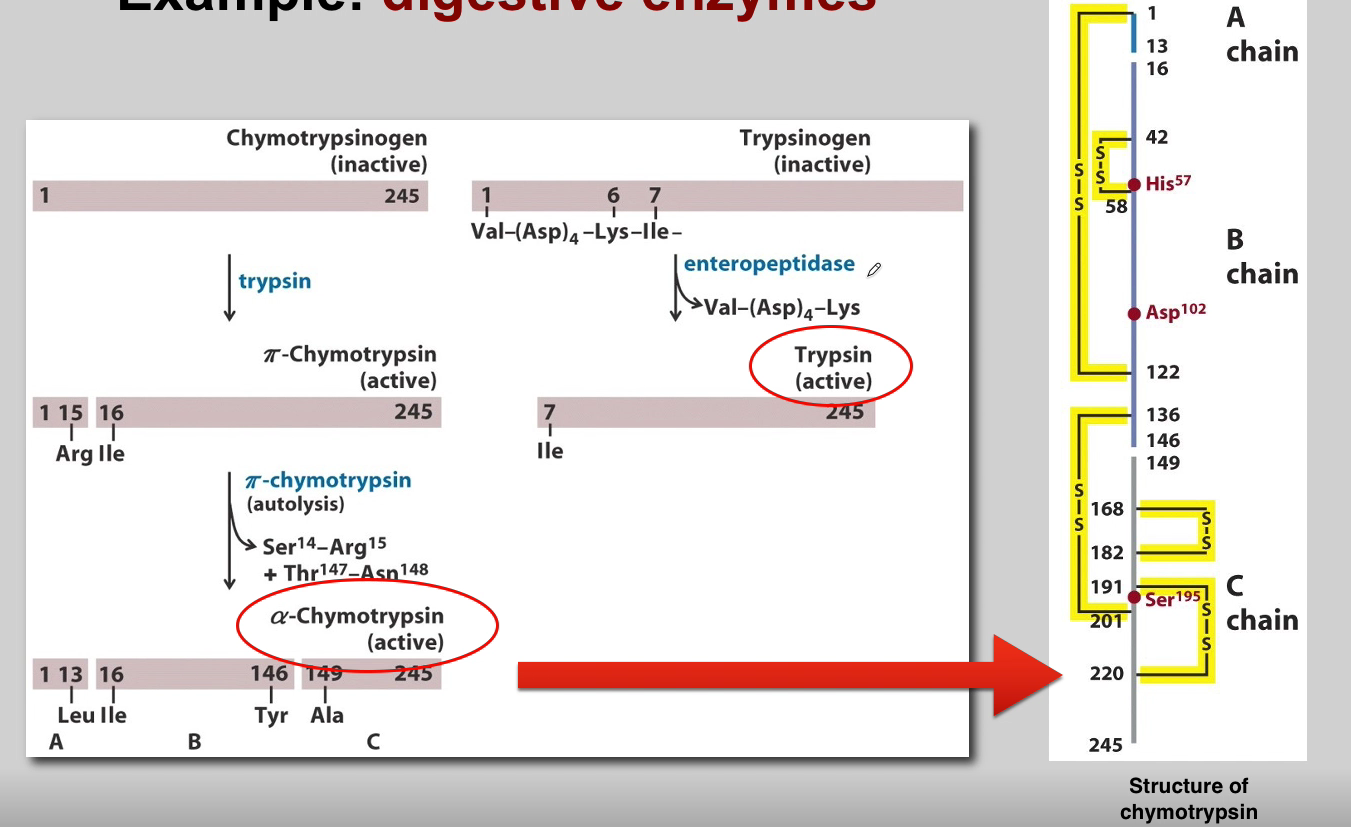
Chymotrypsinogen:
Proenzyme Form: Chymotrypsinogen is the inactive proenzyme (zymogen) of chymotrypsin.
Secreted by Pancreas: Released by the pancreas into the small intestine.
Activation: Converted into active chymotrypsin by proteolytic cleavage.
What is the role of Trypsin? (3)
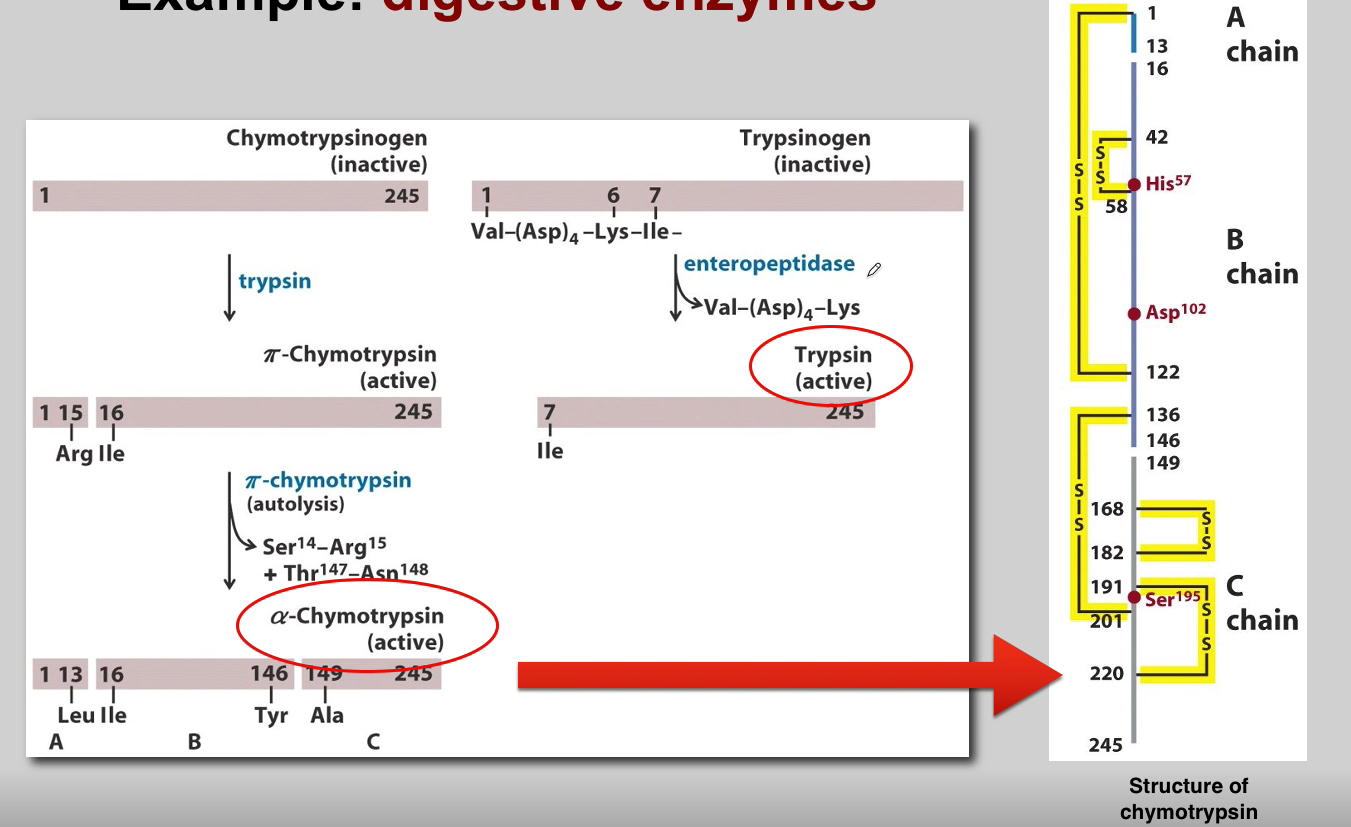
Trypsin:
Activation of Other Zymogens: Acts as an initiator of a cascade of zymogen activations.
Activation of Chymotrypsinogen: Activates chymotrypsinogen to chymotrypsin.
Autocatalysis: Activated trypsin can activate other trypsinogen molecules through autocatalysis.
What is the role of Chymotrypsin? (3)
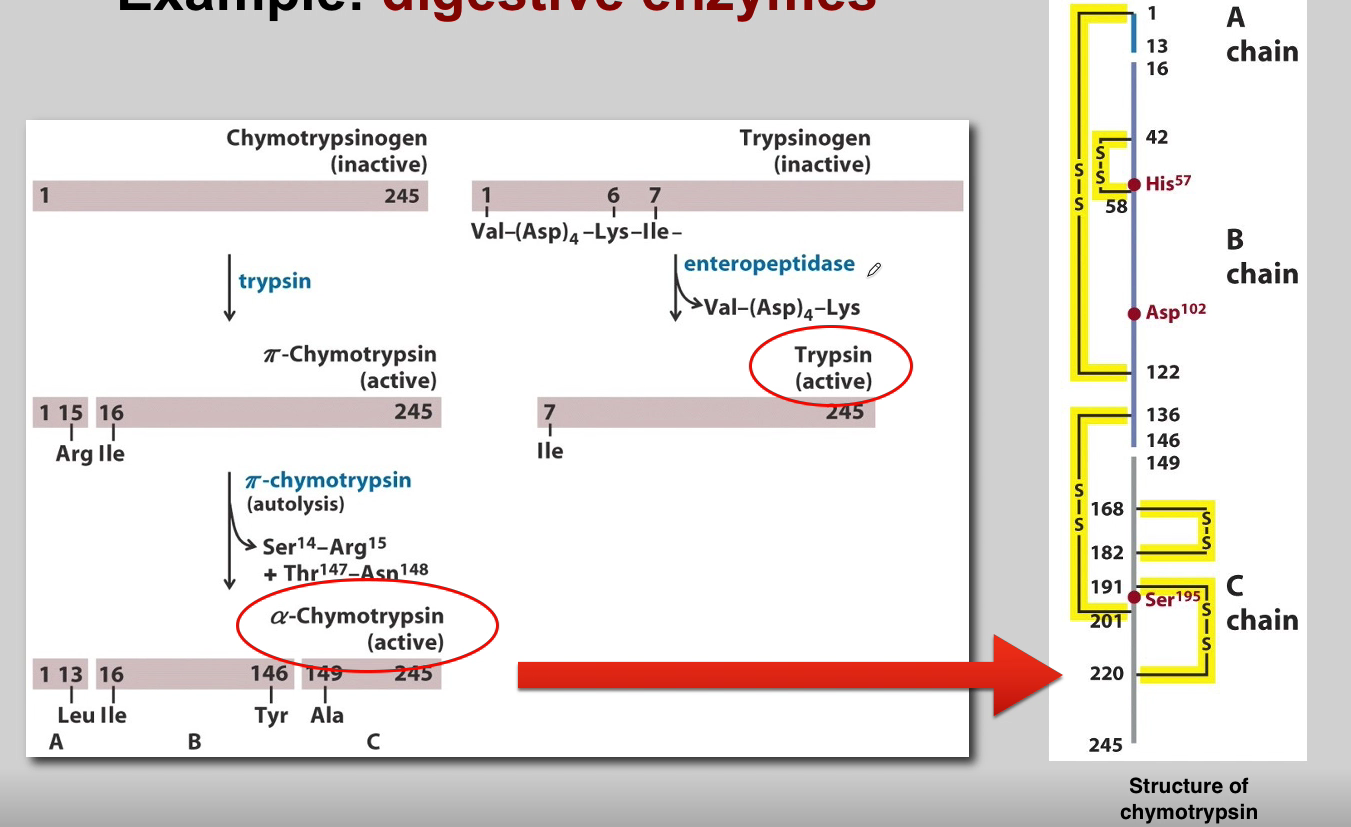
Chymotrypsin:
Proteolytic Activity: Active chymotrypsin is a protease that cleaves peptide bonds.
Substrate Specificity: Targets peptide bonds adjacent to aromatic amino acids.
Role in Digestion: Contributes to the digestion of proteins in the small intestine.
What is the role of Enteropeptidases? (3)
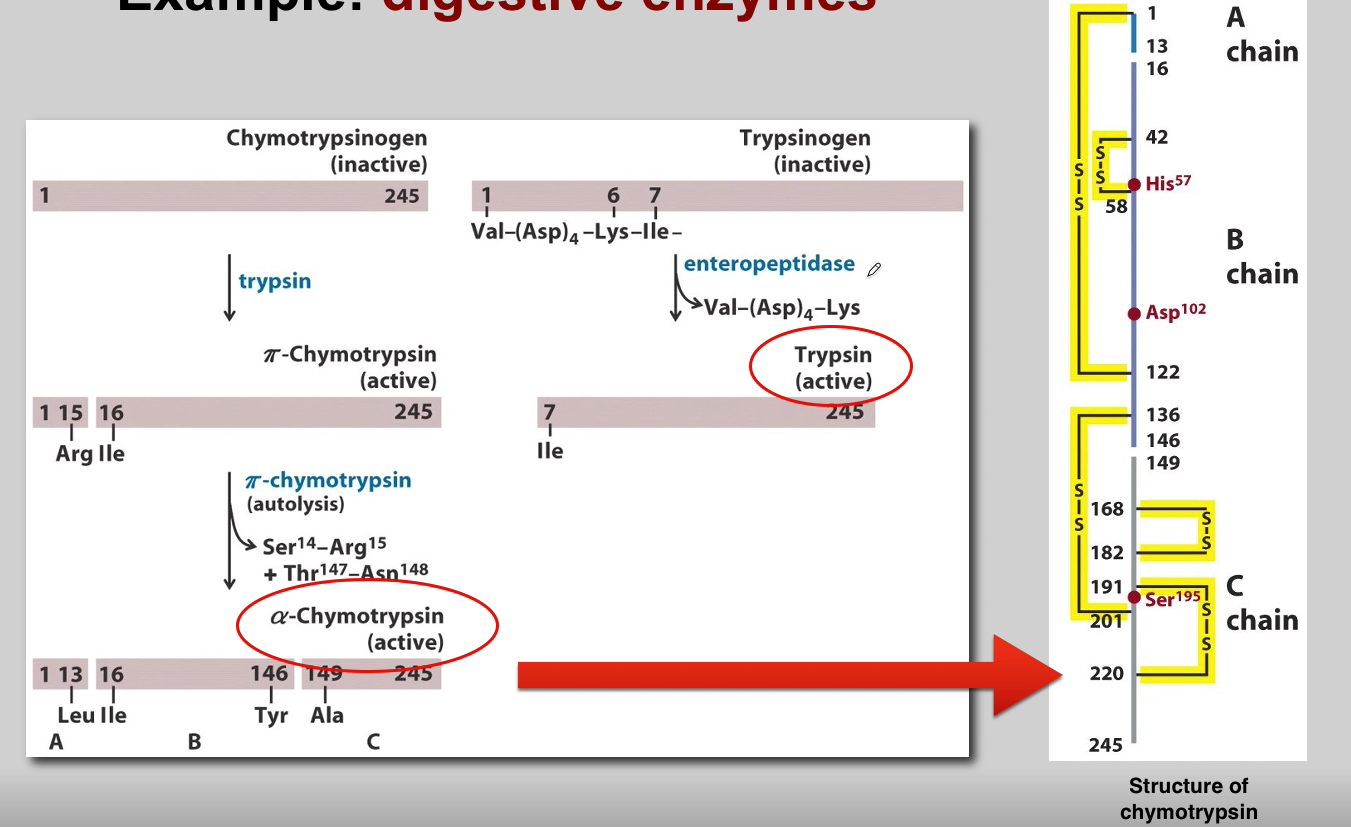
Enteropeptidases (Enterokinase):
Location: Located in the brush border of the small intestine.
Activation of Trypsinogen: Activates trypsinogen to trypsin.
Cascading Effect: Initiates the activation cascade of pancreatic zymogens.
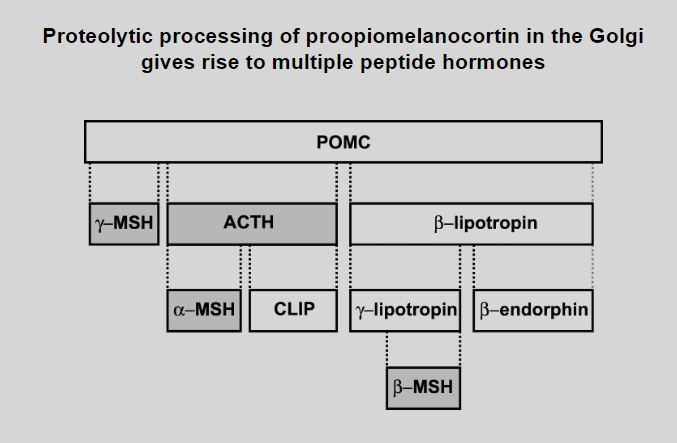
Picture outlining how Proteolytic processing of proopiomelanocortin in the Golgi gives rise to multiple peptide hormones
Insulin is a hormone that must also be processed by?
Proteolysis
Outline the process how proteolysis prepares insulin, from preproinsulin to mature insulin
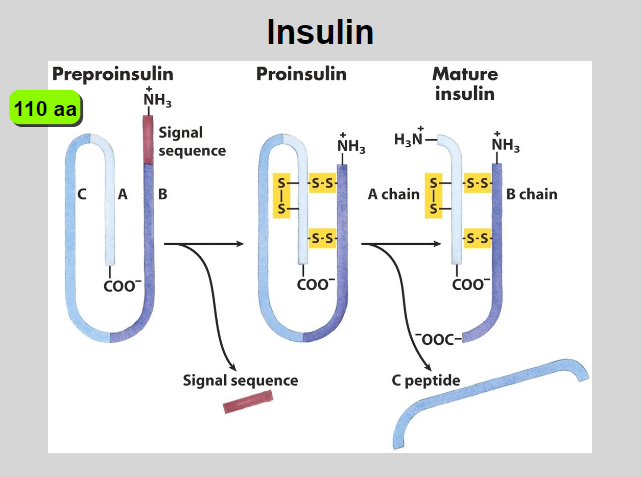
Preproinsulin Synthesis:
Synthesized in the endoplasmic reticulum (ER).
Proinsulin Formation:
Signal peptide removal creates proinsulin with A, B chains, and C peptide.
Transport to Golgi:
Proinsulin transported to the Golgi apparatus.
Proteolytic Cleavage:
Endopeptidases in secretory vesicles cleave C peptide from proinsulin.
Insulin and C Peptide:
Cleavage yields mature insulin and C peptide.
Storage and Release:
Stored in vesicles until stimuli trigger release.
Biological Activity:
Released insulin enters bloodstream, exerting biological effects.
C Peptide Function:
C peptide, though cleaved, has physiological functions.
What is another process that is caused by protein activation by proteolysis?
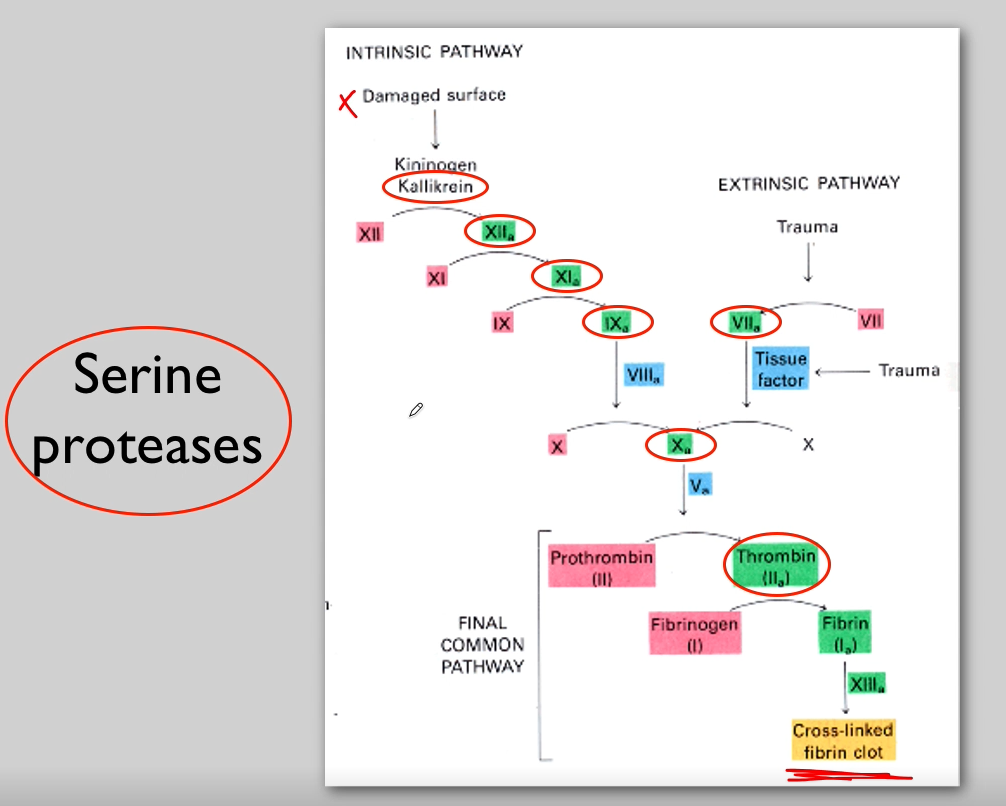
Clotting factors (By serine proteases)
Deficiencies of factor VIII or IX are the cause of what?
X-Linked haemophilia
What is X-linked haemophilia?
Inheritance: X-linked recessive genetic disorder.
Genes Involved: Haemophilia A (Factor VIII deficiency) or Haemophilia B (Factor IX deficiency).
Chromosomal Link: Mutations occur on the X chromosome.
Prevalence: Mainly affects males; females are carriers.
Bleeding Disorder: Impaired blood clotting due to deficient or dysfunctional clotting factors.
Name a famous cysteine protease
-Bromelain (found in pineapples)
-Papain (found in raw papaya fruit)