-
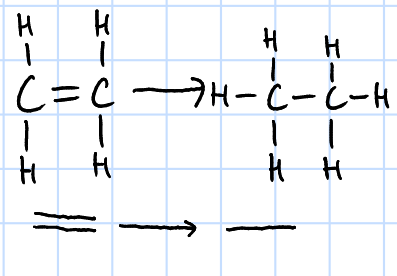
reduction
H2(g), Ni catalyst, heat
-
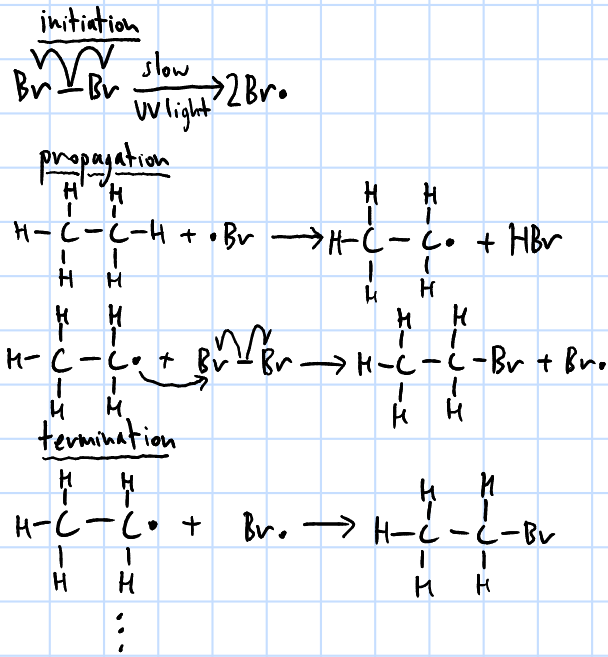
free radical substitution
limited X2(g), UV light
-
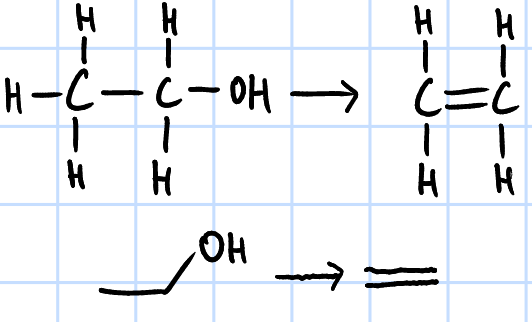
elimination
r&c: concentrated H2SO4, heat OR
Al2O3(s), high temp
-
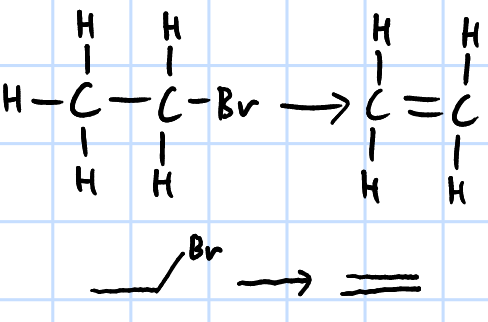
elimination
r&c: KOH/NaOH, ethanol, heat
-
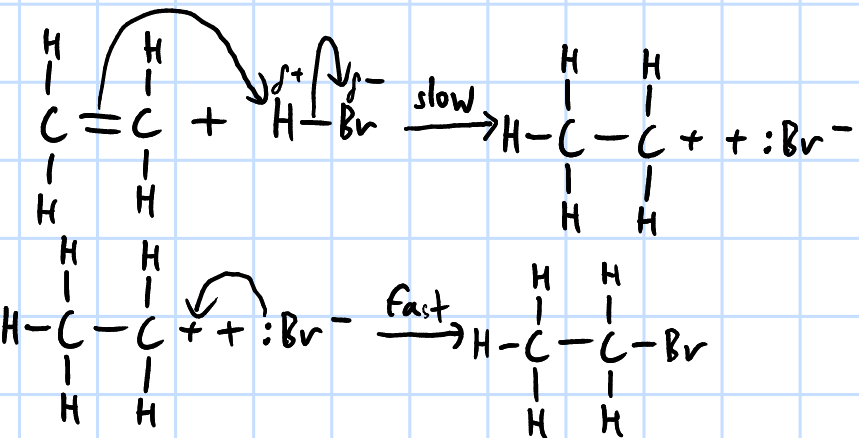
electrophilic addition
r&c: dry HX(g), rtp
-
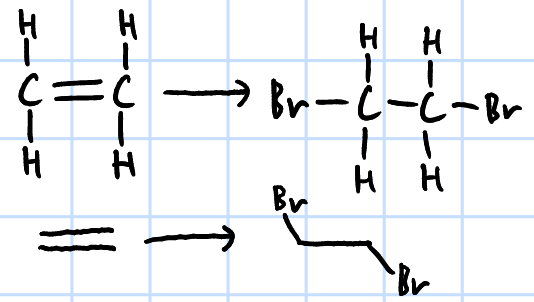
electrophilic addition
r&c: X2 in inert organic solvent(CCl4)
-
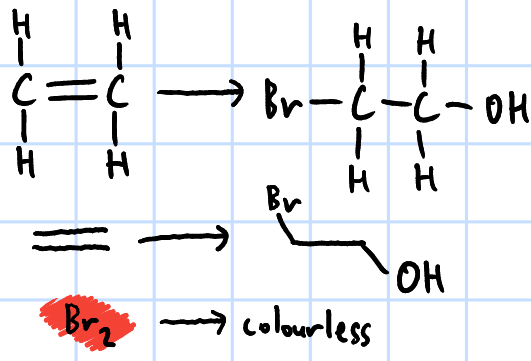
electrophilic addition
r&c: X2(aq)
-
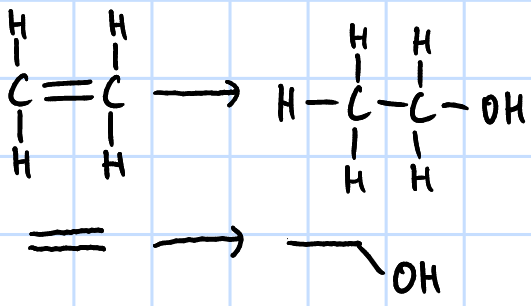
electrophilic addition
r&c: H2O(g), H3PO4 catalyst, high temp & pressure
-
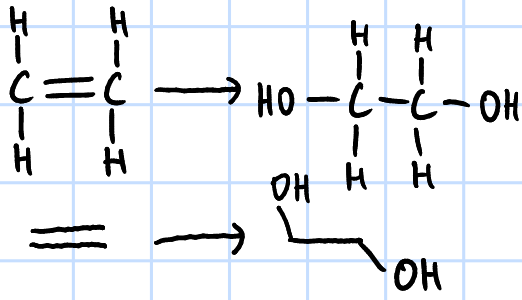
mild oxidation
r&c: KMnO4(aq), NaOH(aq), cold
-
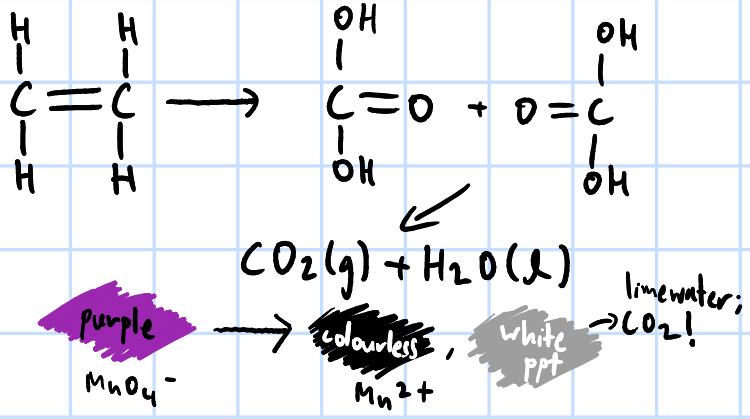
strong oxidation
r&c: KMnO4(aq)/K2Cr2O7(aq), H2SO4(aq), heat
-
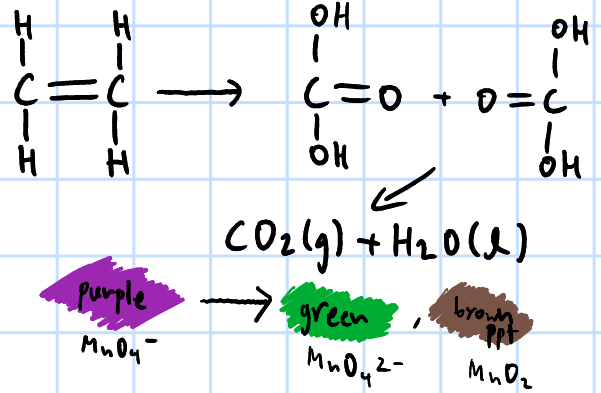
strong oxidation
r&c: KMnO4(aq)/K2Cr2O7(aq), NaOH(aq), heat
-
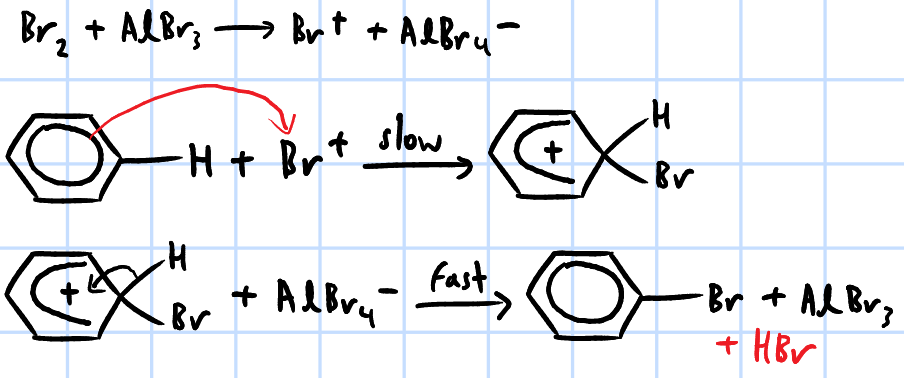
electrophilic substitution
r&c: X2, anhydrous AlX3/FeX3, heat
-
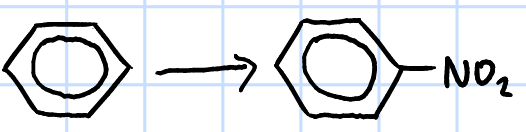
electrophilic substitution
r&c: concentrated HNO3, concentrated H2SO4, 50oC
-
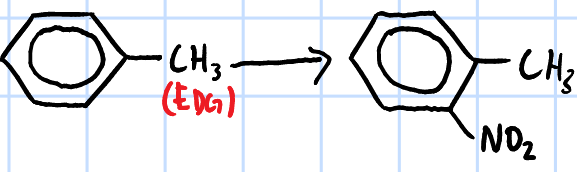
electrophilic substitution
r&c: concentrated HNO3, concentrated H2SO4, <30oC I think
-
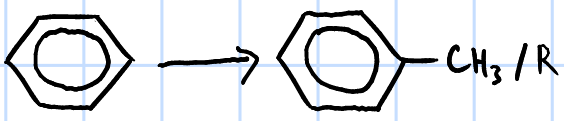
electrophilic substitution(FCA)
r&c: RX, anhydrous AlX3, heat
-
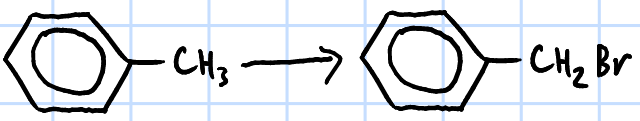
free radical substitution
r&c: limited X2(g), UV light
-
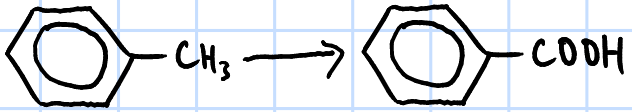
oxidation
r&c: KMnO4(aq), H2SO4(aq), heat
-
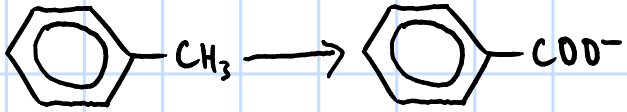
oxidation
r&c: KMnO4(aq), NaOH(aq), heat
-
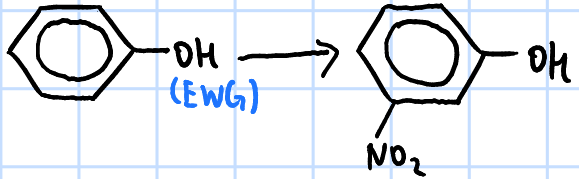
electrophilic substitution
r&c: concentrated HNO3, concentrated H2SO4, >50oC
-
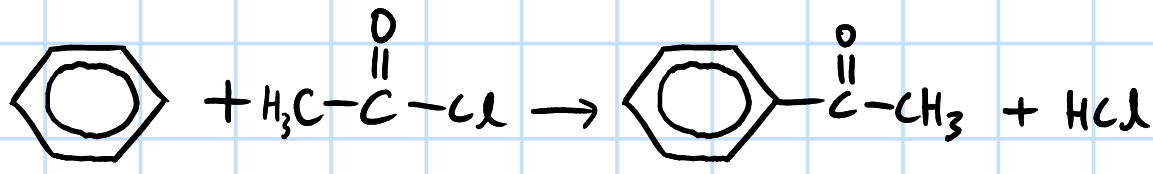
electrophilic substitution(FCA)
r&c: RCOCl, anhydrous AlCl3, heat
-
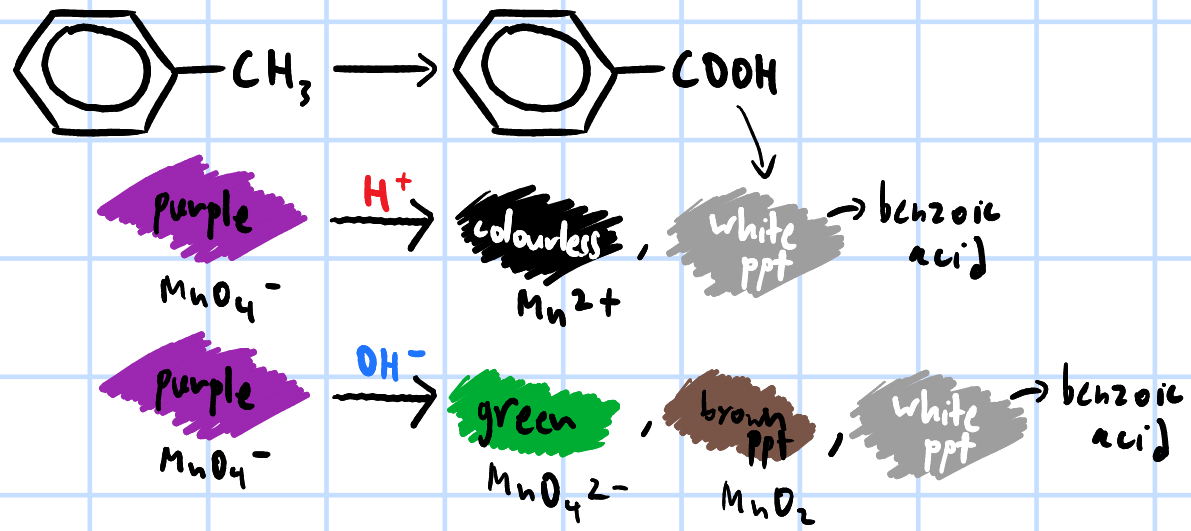
add few drops of KMnO4(aq), H2SO4(aq) & heat each sample separately >
purple KMnO4 turns colourless, white ppt of benzoic acid forms
purple KMnO4 turns green, then brown ppt(Mn2+) & white ppt of benzoic acid forms
-
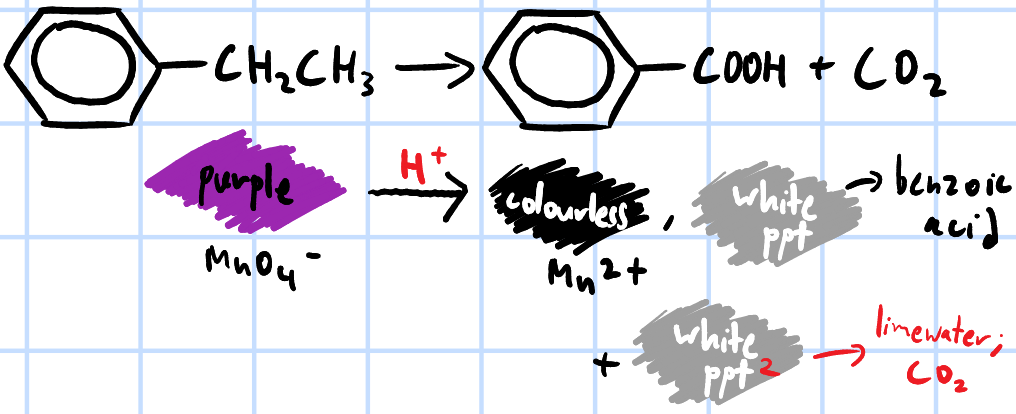
add few drops of KMnO4(aq), H2SO4(aq) & heat both samples separately, pass any gas evolved through limewater >
both decolourise purple KMnO4 and form white ppt of benzoic acid
only ethylbenzene, CO2 gas evolved forms white ppt w/ limewater
-
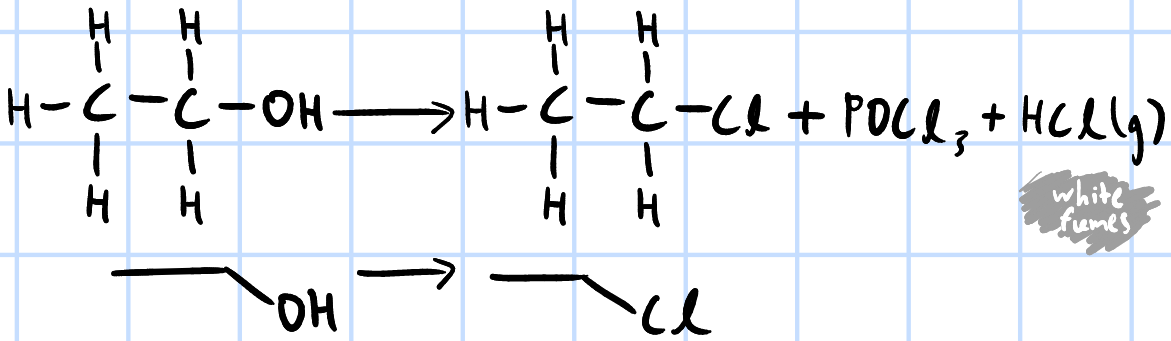
nucleophilic substitution
r&c: PCl5(s), rtp
-
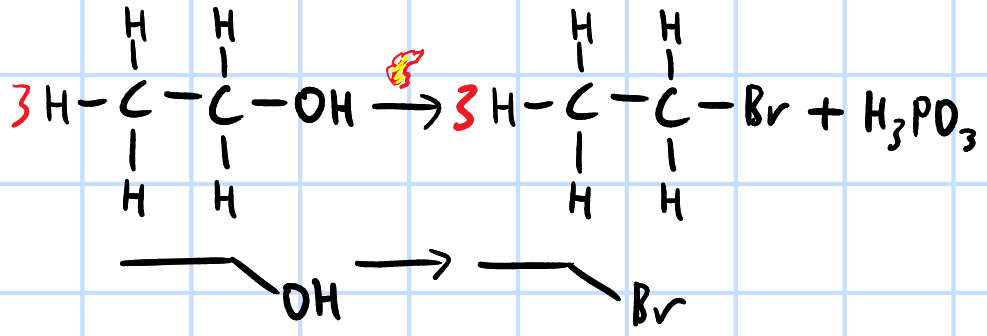
nucleophilic substitution
r&c: PX3(l), heat
-
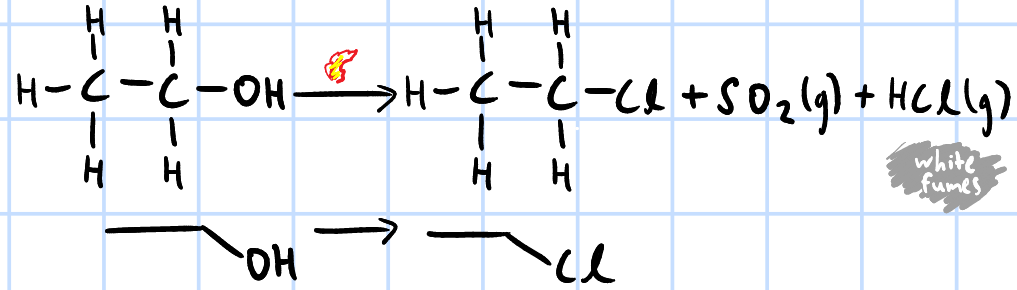
nucleophilic substitution
r&c: SOCl2(l), heat
-
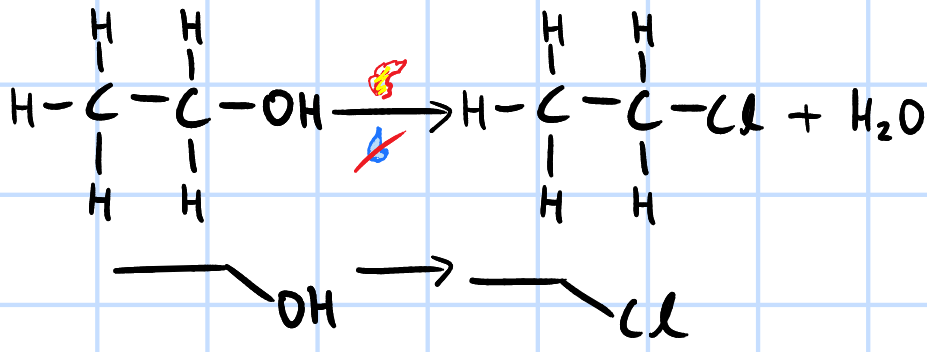
nucleophilic substitution
r&c: dry HX(g), heat
-
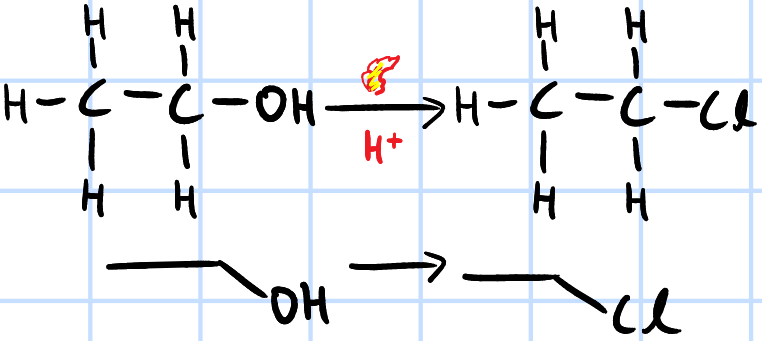
nucleophilic substitution
r&c: concentrated H2SO4, KX(s)/NaX(s), heat
-
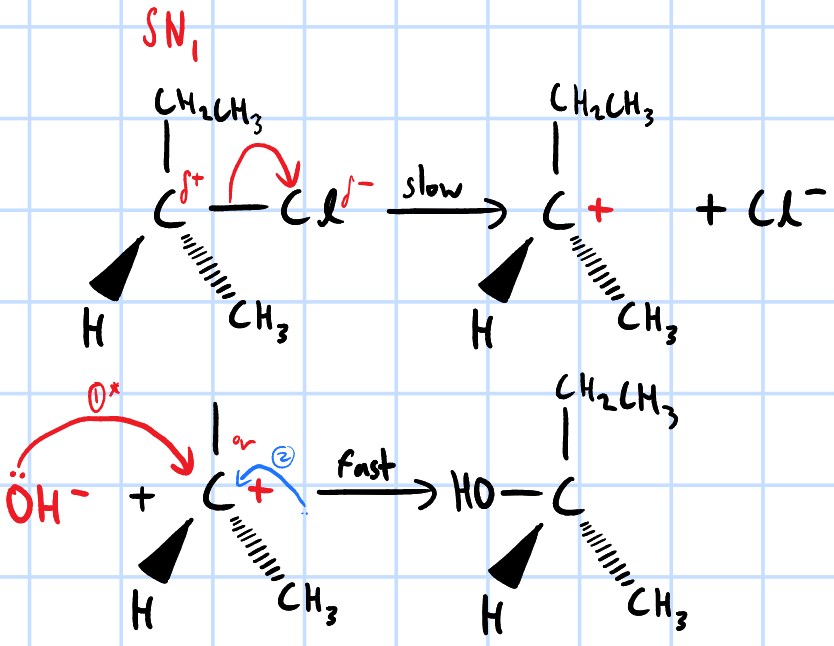
nucleophilic substitution
r&c: NaOH(aq), heat
-
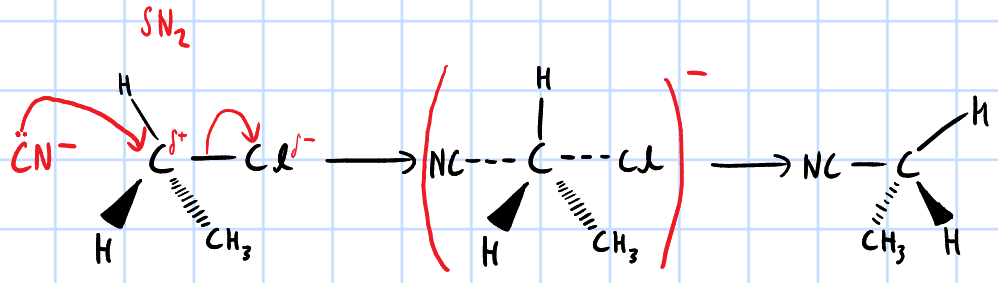
nucleophilic substitution
r&c: NaCN/KCN, ethanol, heat
-
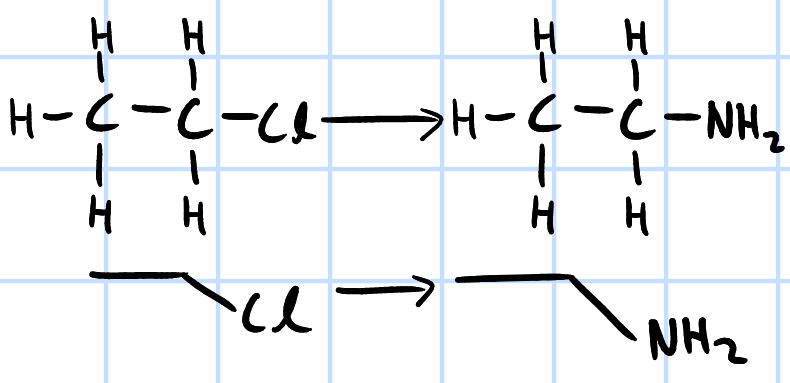
nucleophilic substitution
r&c: excess NH3, ethanol, heat in sealed tube
-
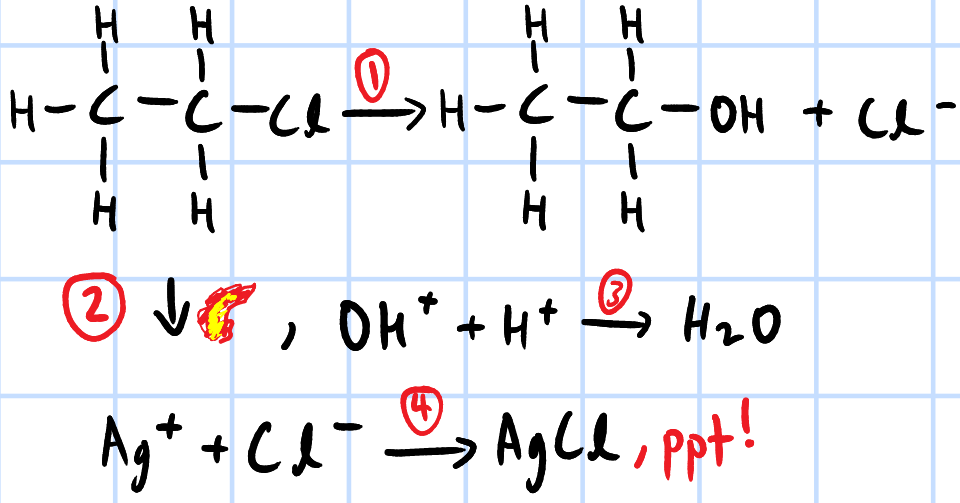
1. NaOH(aq), heat
2. cool mixture, prevent decomposition of AgNO3
3. acidify w/ excess HNO3, neutralise excess NaOH
4. add AgNO3(aq), ppt forms; colour depends on X
-
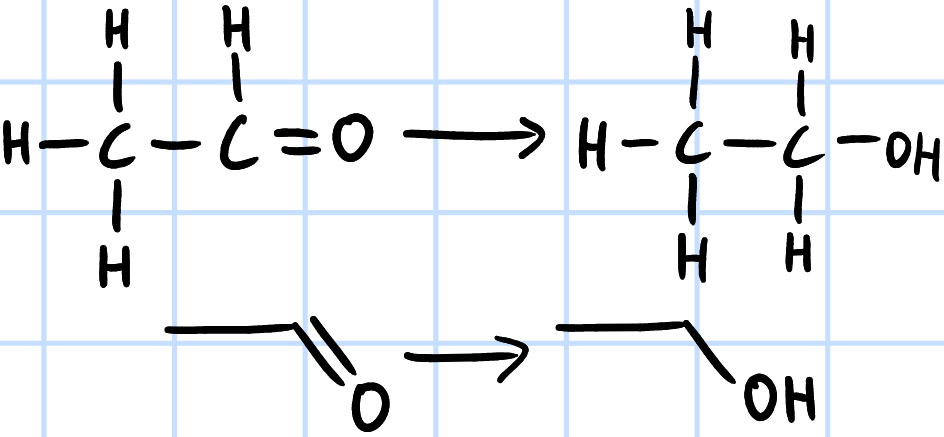
reduction
r&c: LiAlH4, dry ether
NaBH4, methanol
H2(g), Ni catalyst, heat
-
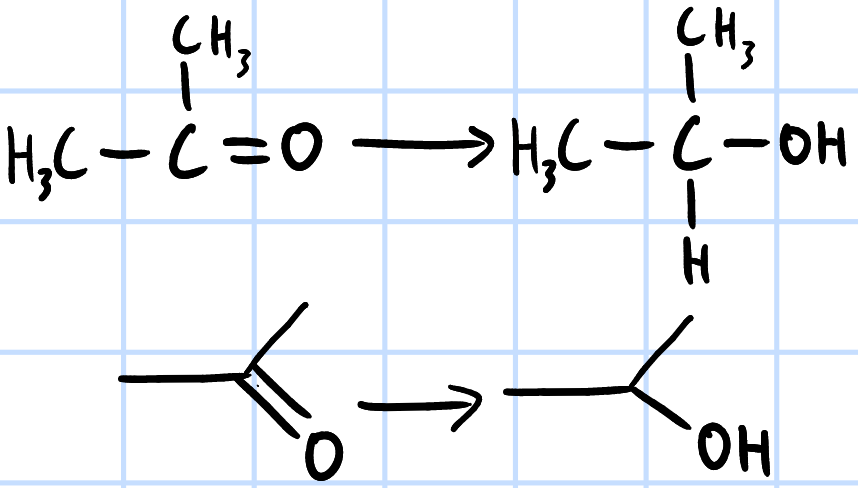
reduction
r&c: LiAlH4, dry ether
NaBH4, methanol
H2(g), Ni catalyst, heat
-
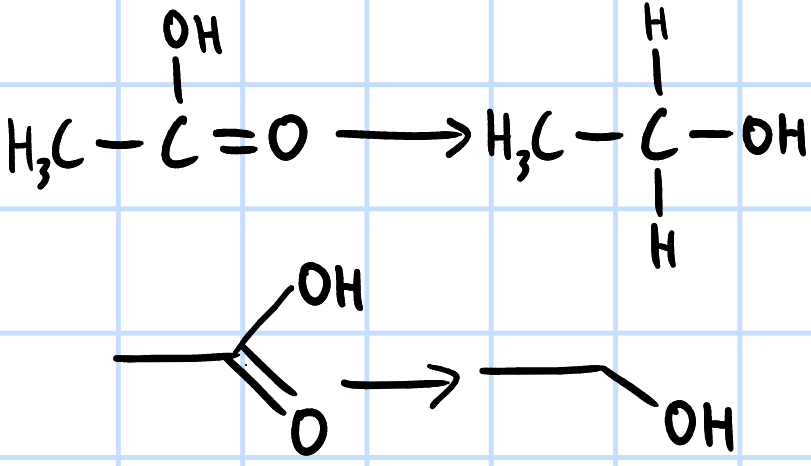
reduction
r&c: LiAlH4, dry ether
-
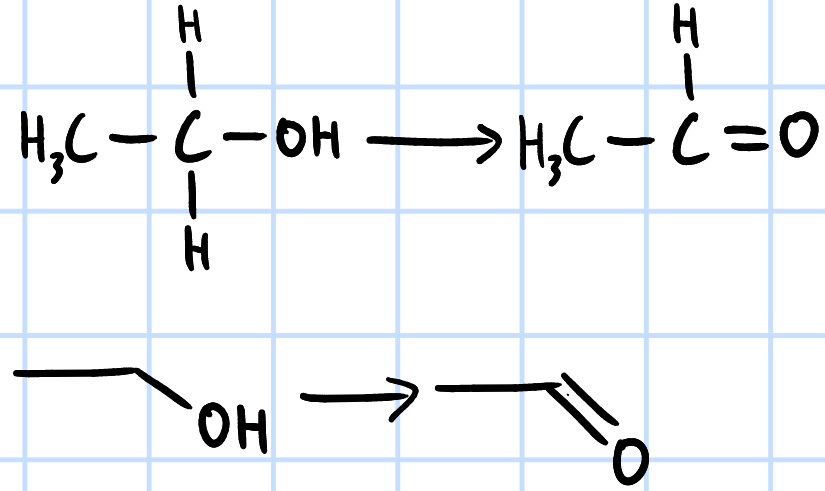
oxidation
r&c: K2CrO7(aq), H2SO4(aq), heat with immediate distillation
-
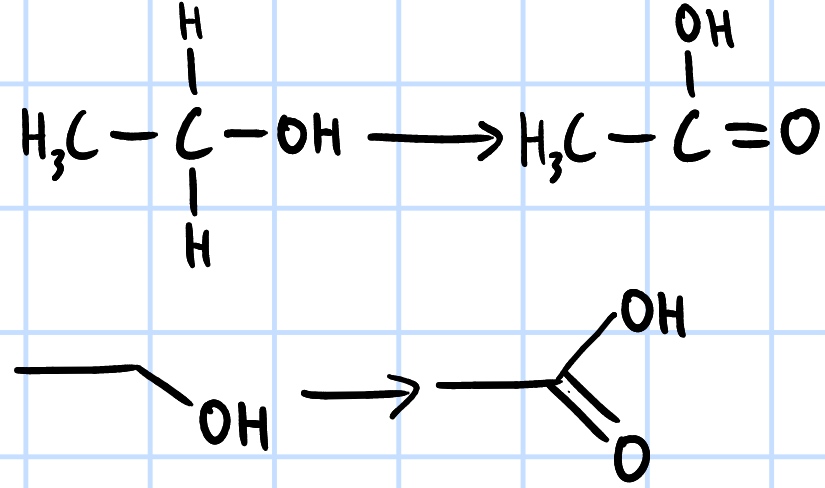
oxidation
r&c: KMnO4/K2CrO7(aq), H2SO4(aq), heat under reflux
-
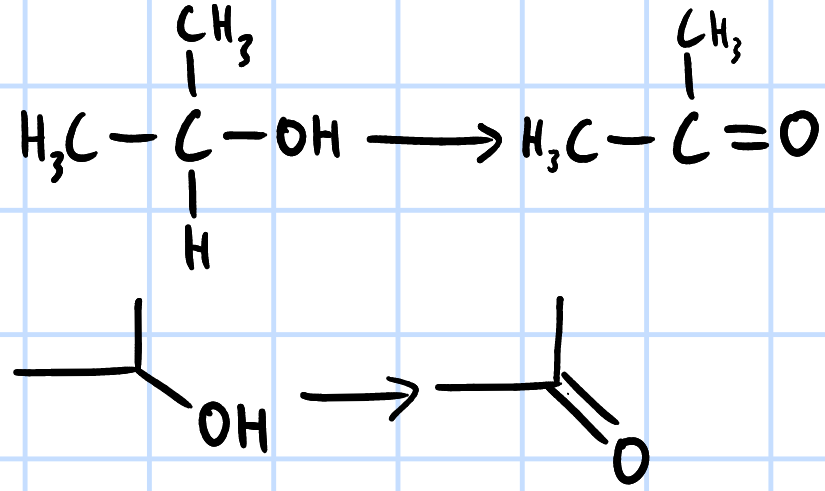
oxidation
r&c: KMnO4/K2CrO7(aq), H2SO4(aq), heat
-

positive triiodomethane/iodoform test
r&c: NaOH(aq), I2(aq), heat
pale yellow ppt chi3
-
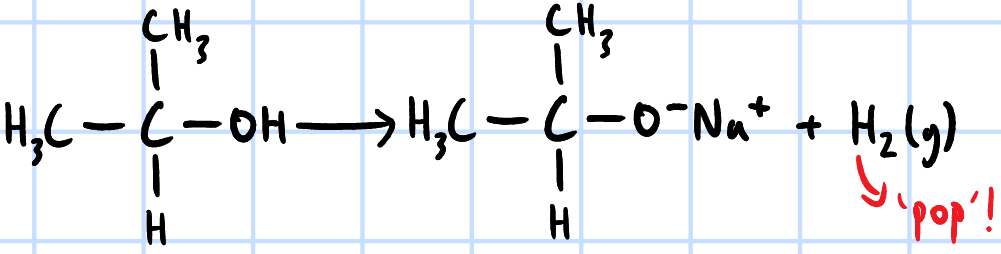
redox reaction
effervescence, lighted splint extinguishes w/ pop sound
-
identify 1o,2o,3o ROH
add PCl5(s) to sample in dry test tube >
white fumes of HCl(g)
-
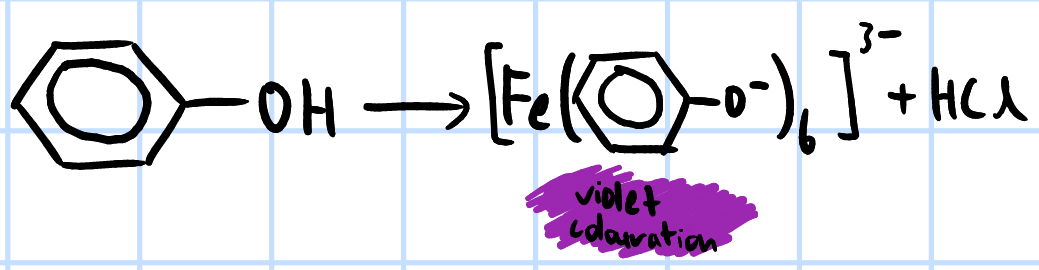
add neutral FeCl3(aq) >
violet colouration observed
-
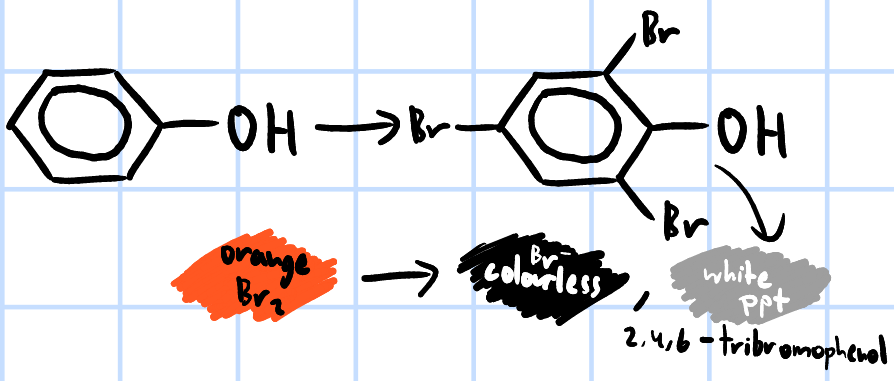
add Br2(aq) >
orange Br2 decolourises initially, white ppt of 2,4,6-tribromophenol forms on excess Br2(aq)
-
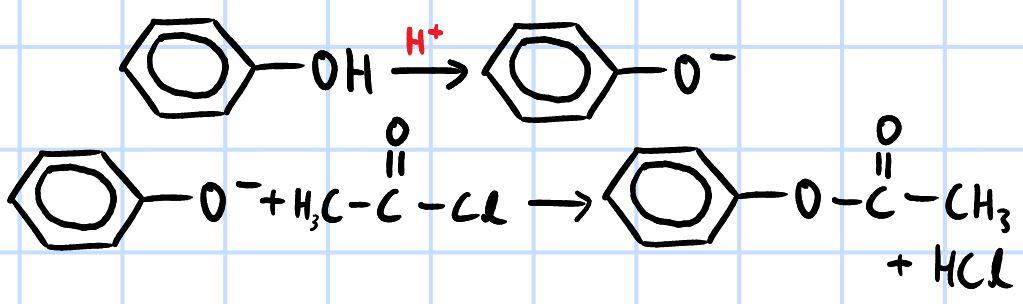
condensation/nucleophilic acyl substitution
r&c: phenol in NaOH(aq), RCOCl, rtp
-
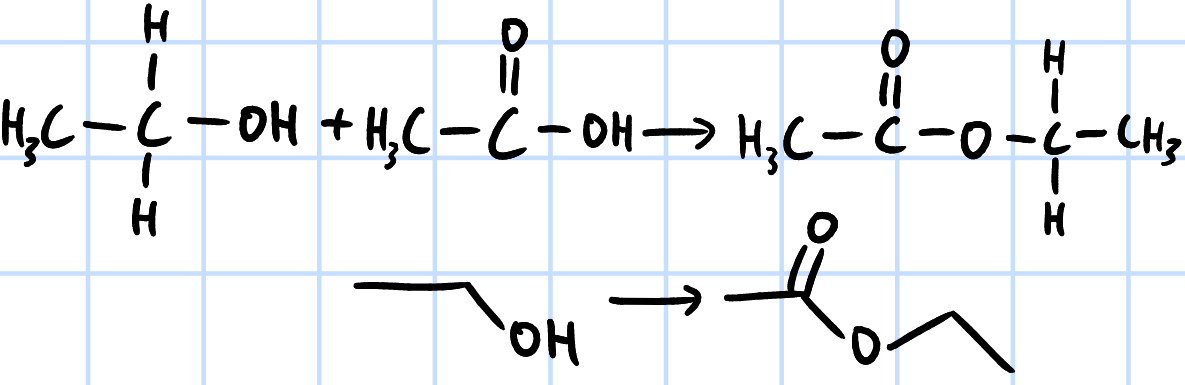
condensation/nucleophilic acyl substitution
r&c: RCOOH, concentrated H2SO4, heat
-
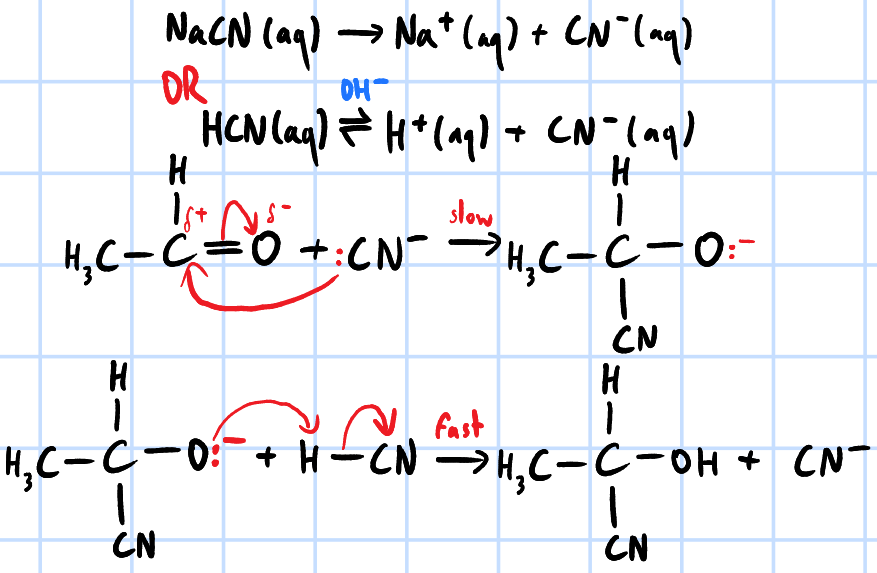
nucleophilic addition
r&c: HCN, trace amounts of NaCN/trace amounts of NaOH, cold
-
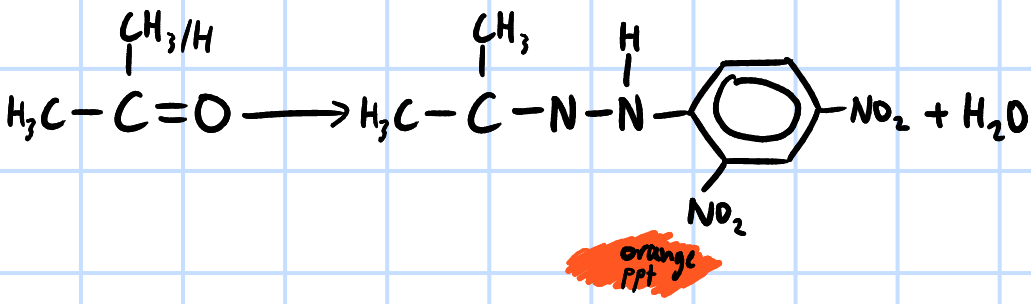
condensation
r&c: 2,4-dinitrophenylhydrazine
orange ppt
-
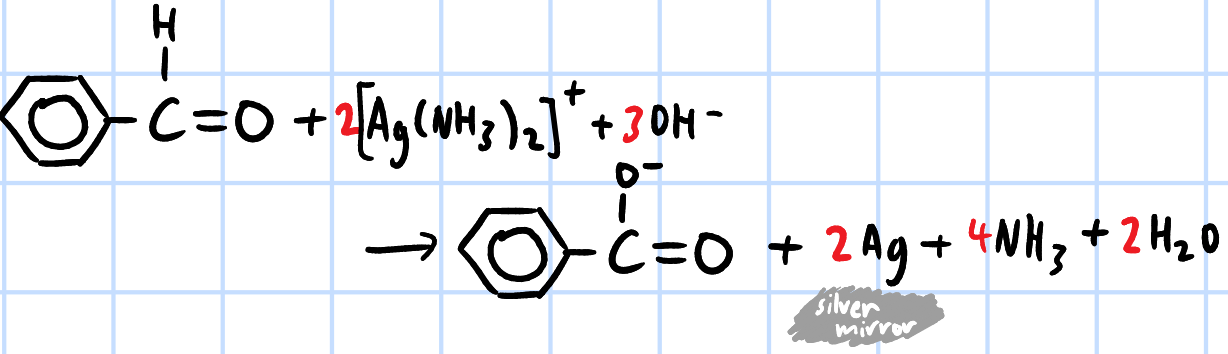
oxidation
r&c: Tollen's reagent([Ag(NH3)2]+(aq), heat
silver mirror
-
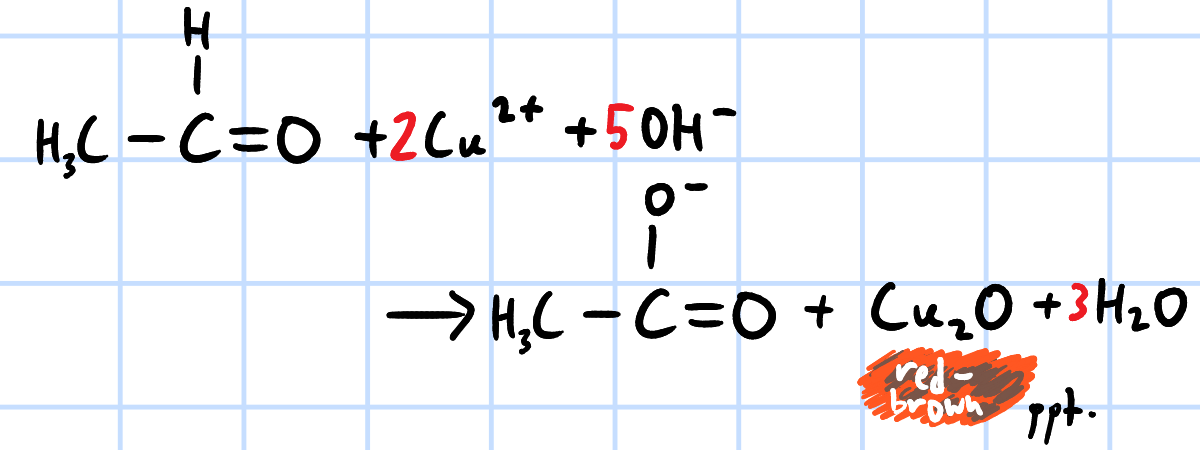
oxidation
r&c: Fehling's solution(alkaline Cu2+ complex), heat
red brown ppt of Cu2O
-
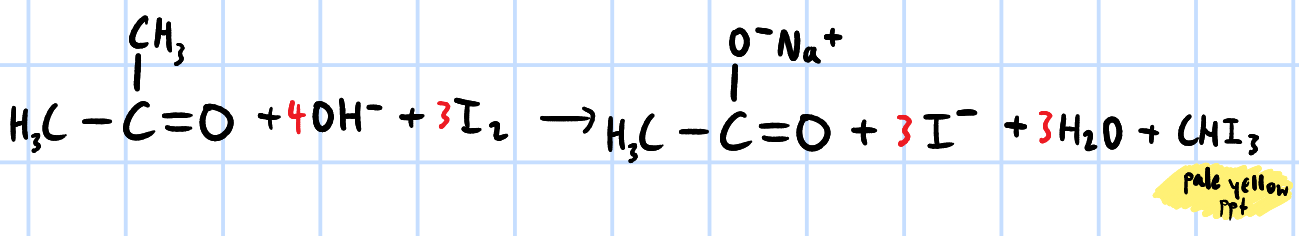
positive triiodomethane/iodoform test
r&c: NaOH(aq), I2(aq), heat
pale yellow ppt chi3
-
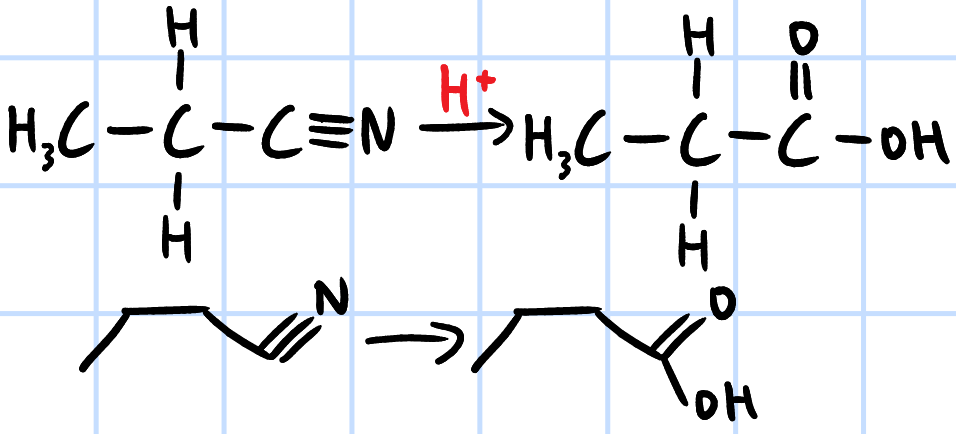
acid hydrolysis
r&c: H2SO4(aq), heat
-
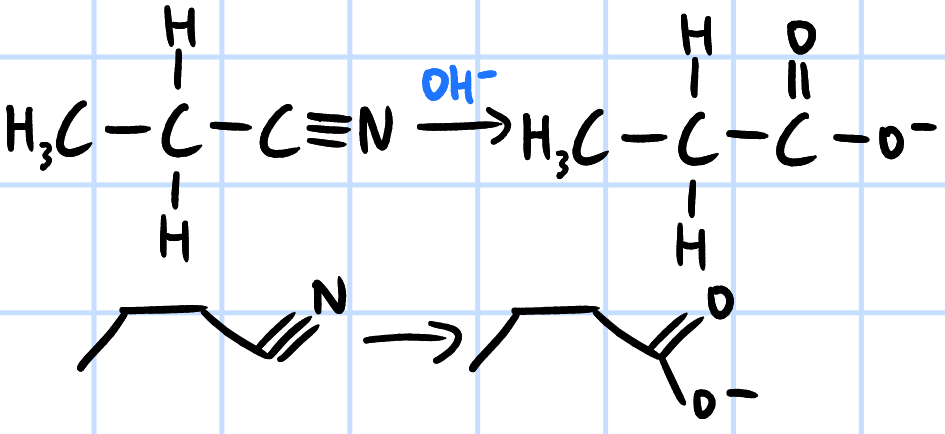
alkaline hydrolysis
r&c: NaOH(aq), heat then
HCl/H2SO4(aq) to get RCOOH
-
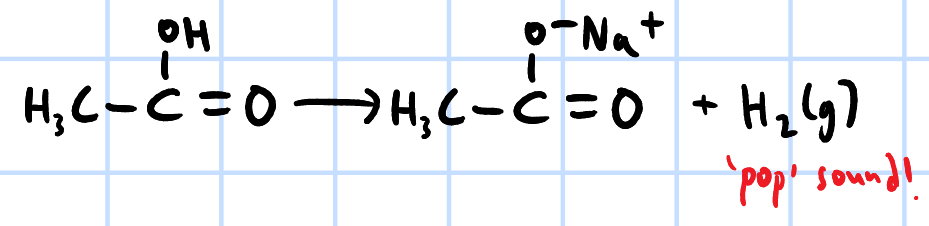
redox
r&c: Na/K/Mg
effervescence, H2(g) evolved extinguishes lighted splint w/ pop sound
-
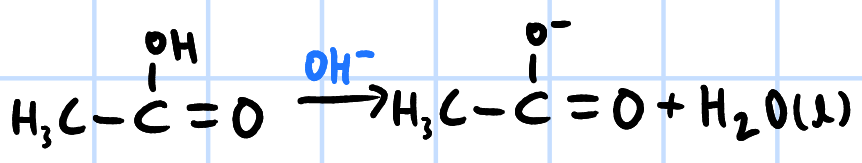
acid-base reaction
r&c: NaOH/KOH/NH3(aq)
-
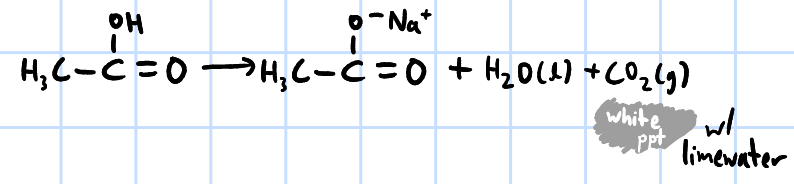
acid-base reaction
r&c: Na2CO3(aq)/NaHCO3(aq)
effervescence, CO2(g) evolved forms white ppt when passed through limewater
-
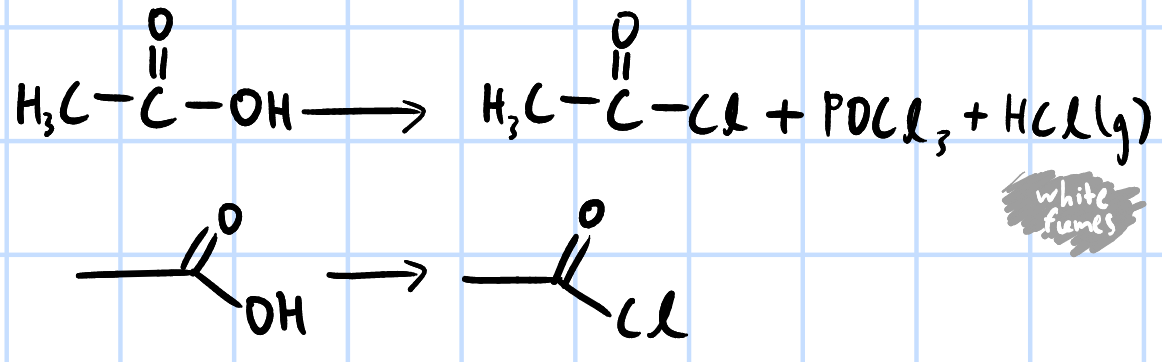
nucleophilic acyl substitution
r&c: PCl5, rtp
-
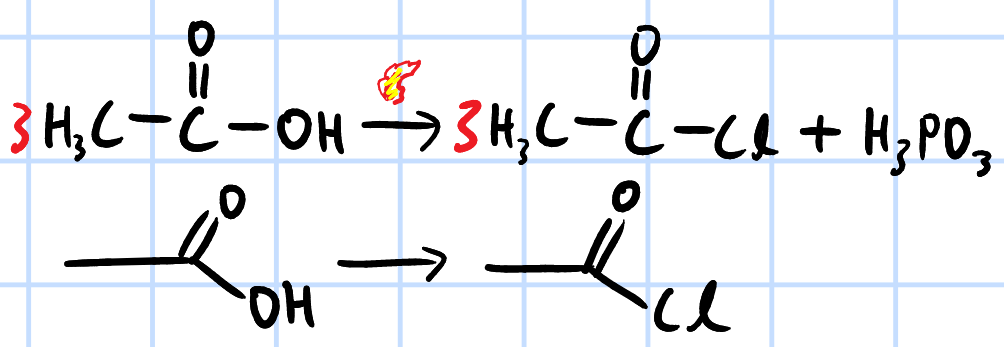
nucleophilic acyl substitution
r&c: PCl3, heat
-
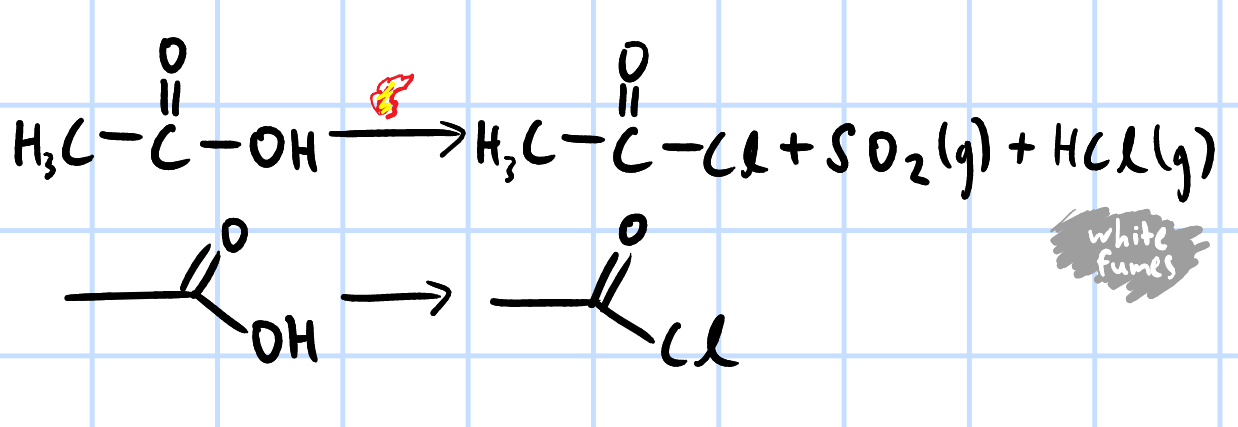
nucleophilic acyl substitution
r&c: SOCl2, heat
-
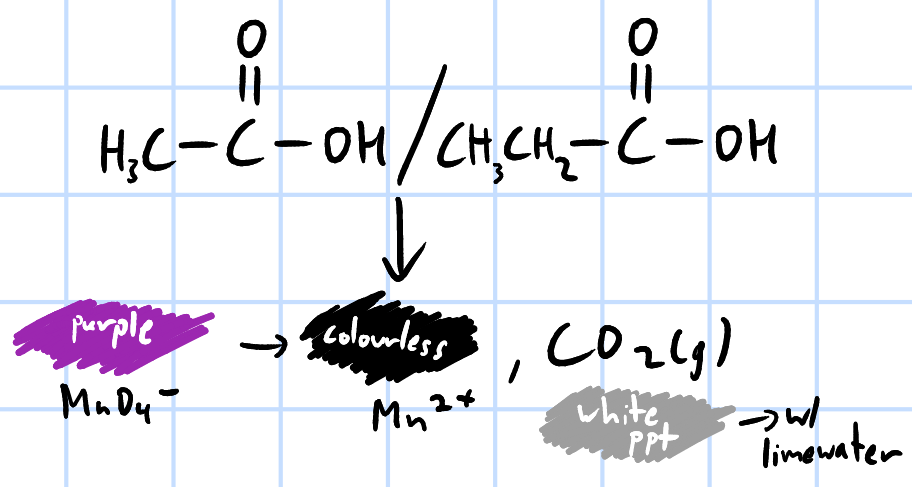
oxidation
r&c: KMnO4(aq), H2SO4(aq), heat under reflux
purple KMnO4(aq) decolourises, effervescence of CO2, forms white ppt w/ limewater
-
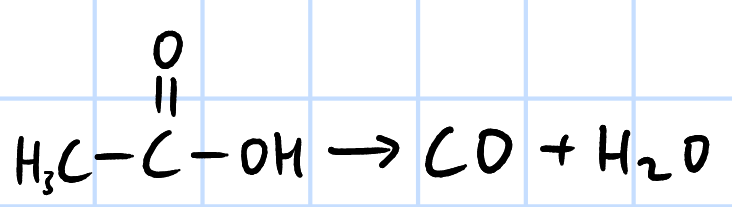
elimination
r&c: concentrated H2SO4, heat
-
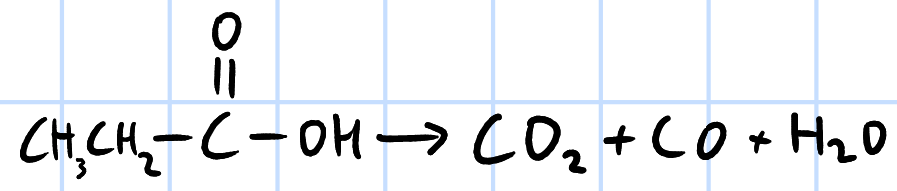
elimination
r&c: concentrated H2SO4, heat
-
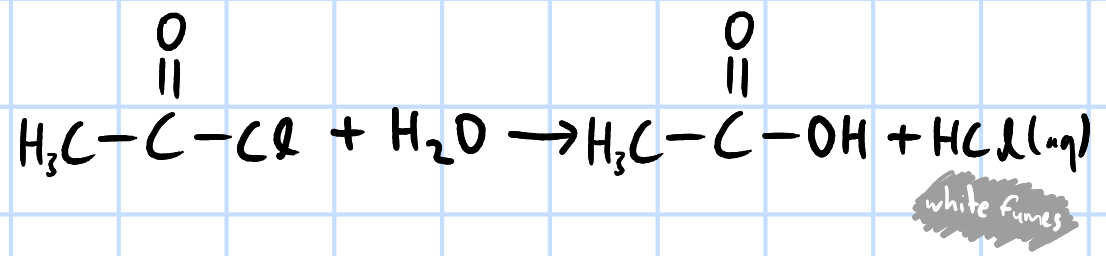
hydrolysis/nucleophilic acyl substitution
r&c: water, rtp
-
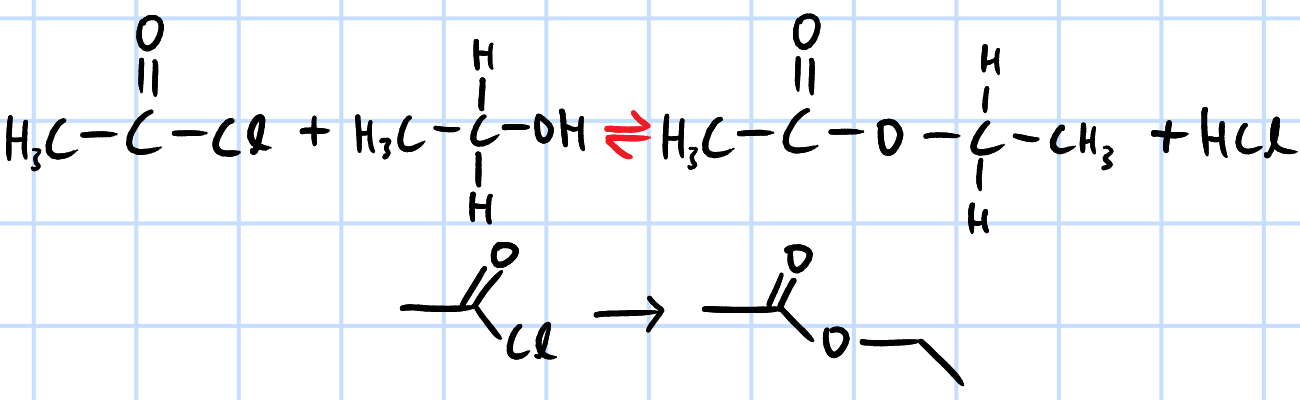
condensation/nucleophilic acyl substitution
r&c: aliphatic ROH, rtp
-
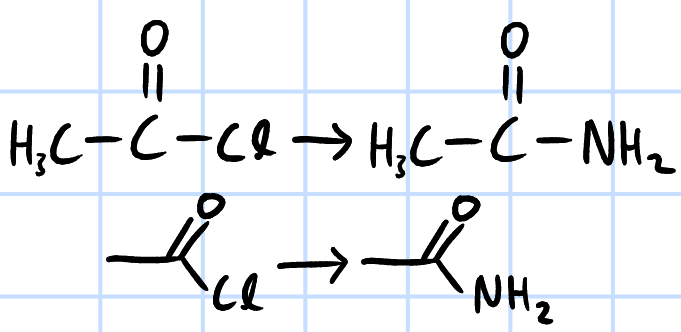
condensation/nucleophilic acyl substitution
r&c: NH3, rtp
-
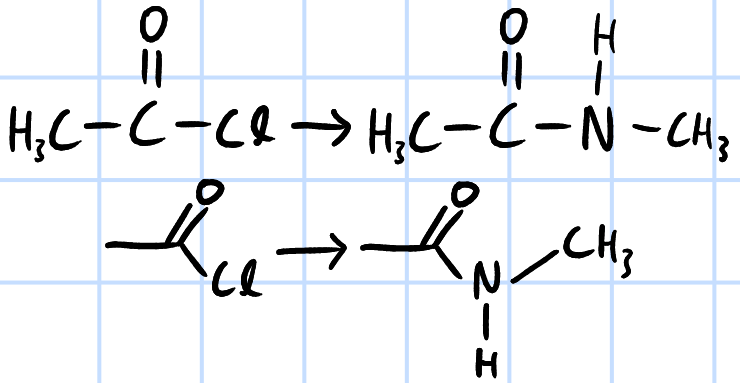
condensation/nucleophilic acyl substitution
r&c: RNH2, rtp
-
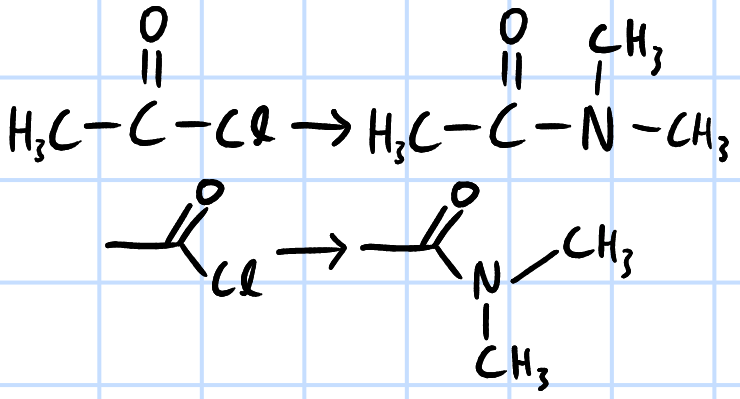
condensation/nucleophilic acyl substitution
r&c: RNHR', rtp
-
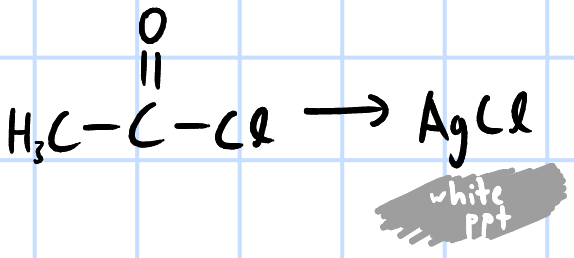
add AgNO3(aq) to test tube containing RCOCl >
white ppt of AgCl formed immediately
-
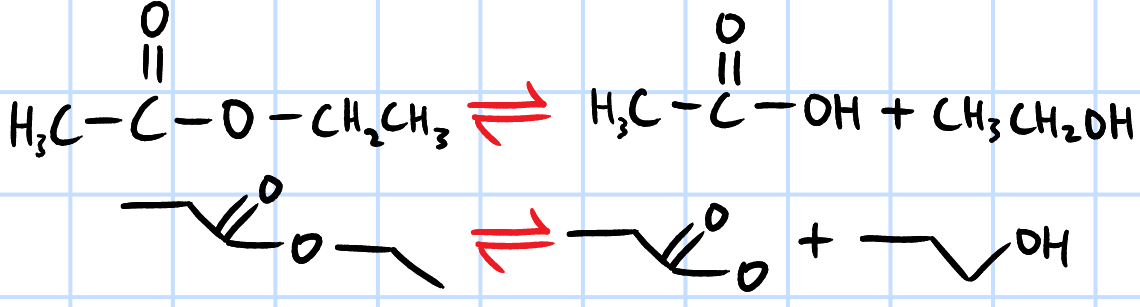
acid hydrolysis
r&c: H2SO4(aq), heat
-
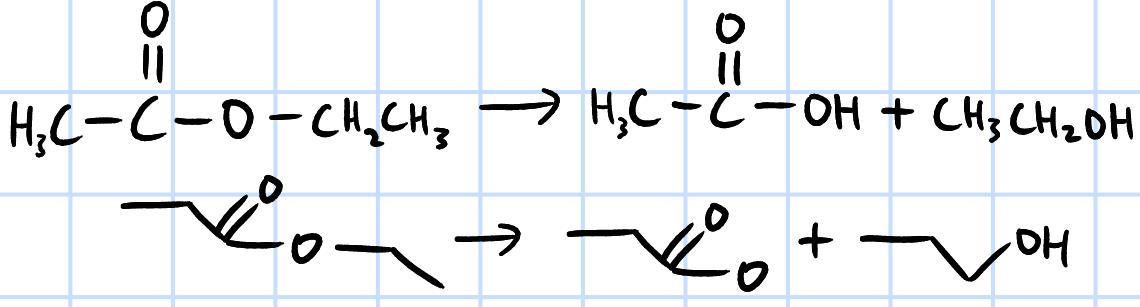
alkaline hydrolysis
r&c: NaOH/KOH(aq), heat
-
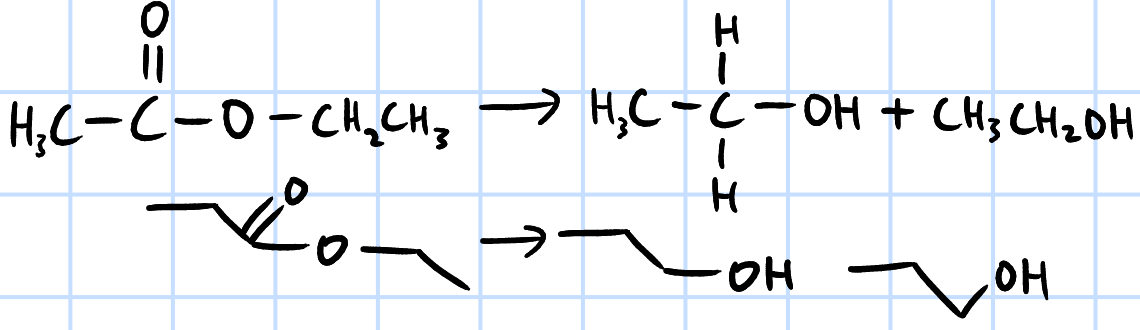
reduction
r&c: LiAlH4, dry ether
-
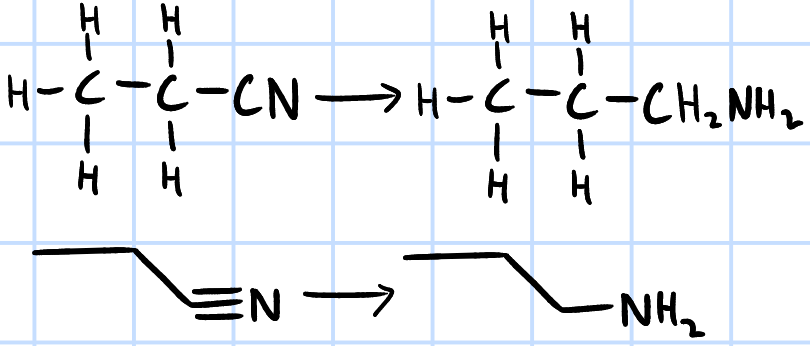
reduction
r&c: LiAlH4, dry ether
H2(g), Ni catalyst, heat
-
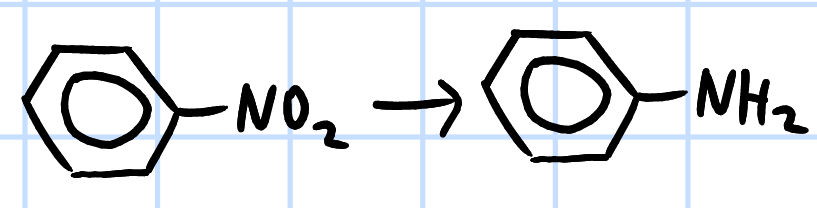
reduction
r&c: Sn, concentrated HCl, heat then
NaOH(aq)
-
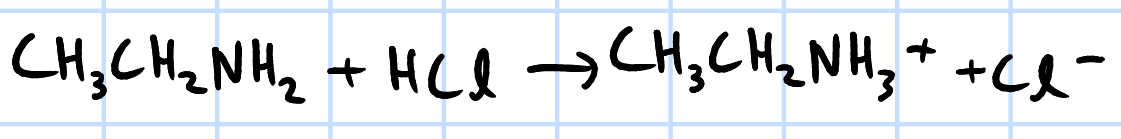
acid-base reaction
H2SO4/HCl(aq), rtp
-
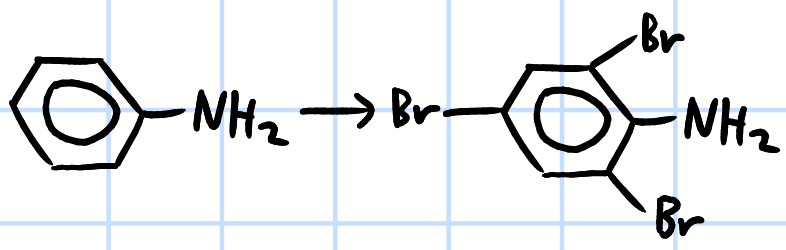
electrophilic substitution
r&c: Br2(aq), rtp
-
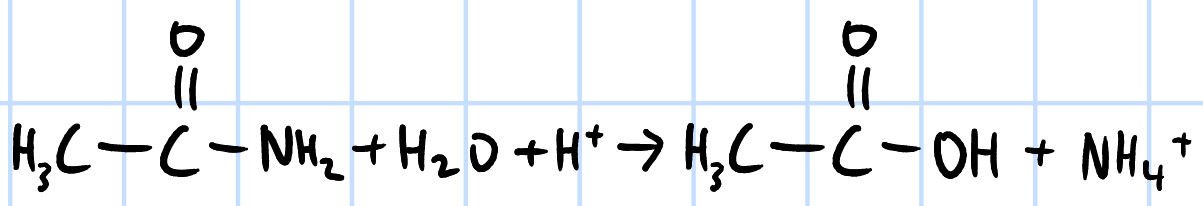
acid hydrolysis
r&c: H2SO4/HCl(aq), heat under reflux
-
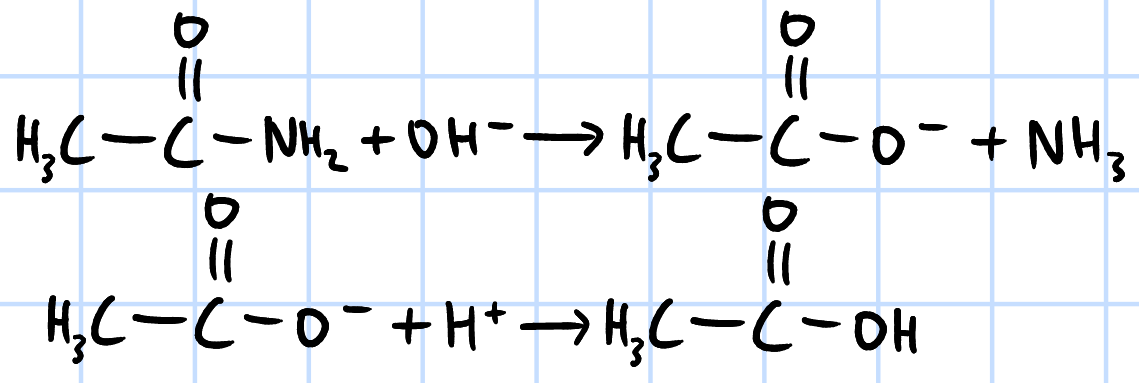
alkaline hydrolysis
r&c: NaOH(aq), heat under reflux then
H2SO4/HCl(aq), rtp
-
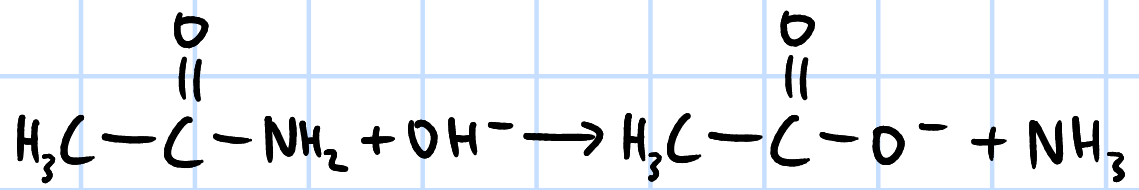
alkaline hydrolysis
r&c: NaOH(aq), heat under reflux
-
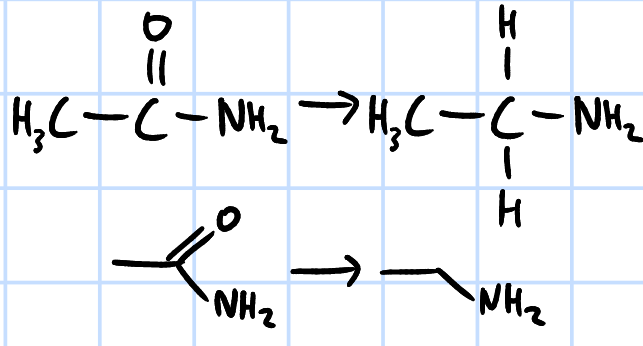
reduction
r&c: LiAlH4, dry ether
-
hydrolysis proteins
acid hydrolysis
r&c: H2SO4(aq), heat under reflux for a long time

