-
Are electromagnetic waves transverse or longitudinal?Transverse
-
What phenomena can be used to show that light behaves as a particle?The photoelectric effect
-
Describe the photoelectric effect.When light above a particular frequency is shone on metal, electrons are released - these released electrons are 'photoelectrons'
-
What is the threshold frequency?The minimum frequency of light required for an electron to be emitted.
-
What equation is used to determine the energy of a photon?E = hf = hc/wavelength
-
What does a photon need to be of a minimum frequency to liberate an electron?The energy of the photon is determined by its frequency, the photon's energy must be greater than the work function (energy needed to break bonds holding the electron) in order for an electron to be emitted
-
If a photon has a frequency higher than the threshold frequency, what would occur?The elctron will be liberated and the remaining energy is the kinetic energy of the electron
-
If light is incident on a metal and photoelectric emission does not occur, what is the effect of increasing light intensity?If it is more intense the there would be more photons incident on the metal each second However each photon still carries the same amount of energy as before Therefore it still does not contain enough energy to liberate an electron No effectt
-
What is the photoelectric equation?
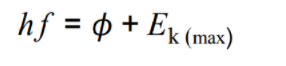 work funtion + maximum kinetic energy of the photoelectrons
work funtion + maximum kinetic energy of the photoelectrons -
Define the work functionThe energy required by an electron to overcome the metallic bond holding it in the metal
-
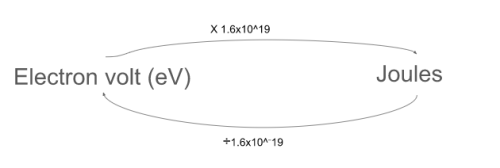
What is an electron volt?The kinetic eergy of an electron that has been accelerated from rest through a potential difference of 1V
-
How to convert eV to J
-
How does a flourescent tube work?High voltge applied across mercury vapour accelerates fast moving free electrons which collide with the mercury atoms Mercy electrons are excited and then return to the ground state, releasing a UV photon The tube's phosphorus coating absorbs the UV photons and its electrons are excited, they cascade down the energy levels ad emit visible light photons
-
What can be used as evidence for the discrete energy levels in atoms?Line emissions and absorption spectra as the lines appear at discrete points which show where a light photon of specific frequency and wavelength has been absorbed or emitted, this shows electrons can only absorb an exact amount of energy to be excited to the next discrete energy level
-
What is wave particle duality?All particles have both particle and wave properties, waves can have particle properties, e.g. light acts as a particle in the photoelectric effect and as a wave when it is diffracted
-
What is the equation for de Broigle wavelength?wavelength = h/mv mv is momentum
-
explaination of experimental findings from when UV light is shown on negatively charged gold leaf experiment why electrons are emitted instantlyAs soon as a photon strikes an electron, the electron has enough energy to escape (if the frequency is high enough). This can happen as soon as the light is switched on.
-
explaination of the experimental findings from when UV light is shown on negatively charged gold leaf experiment why increasing the intensity of light increases the number of electrons emitted not their kinetic energiesIntensity of light depends on the number of photons. The more photons, the greater the intensity. The more photons, the greater the chances of colliding with electrons and so the more electrons are released.
-
explaination of the experimental findings from when UV light is shown on negatively charged gold leaf experiment why light needs to be a certain frequencyAs E= hf energy of the photon is connected to its frequency and so only photons of high enough energy will be able to release electrons from the metal, so unless the frequency of the photon is hgh enough, its energy will not be enough to release an electron
-
explaination of the experimental findings from when UV light is shown on negatively charged gold leaf experiment why KE of electrons depend on the frequencyEnergy of the photons is E = hf and this is completely transferred to the electrons during the collision, so the kinetic energy of the electron depends directly on this frequency
-
conditions for excitation using photonsexcitation will only occur if the photon's energy is exactly equal to the difference in energy of the initial and final energy level
-
How can electrons move down energy levels?Emitting a photon
-
wavelength of red light, blue light,microwaveRed light ~ 700nmBlue light ~ 400nmMicrowave ~ 1 cm
-
Hertz experiment conclusionsShowed that waves Travelled in straight linesCould be reflected from a metal mirrorCould be polarisedCould refract through a prism
-
can electrons emitted from the plate come backElectrons that escape from the metal plate can be attracted back to it by giving the plate a sufficient positive charge.
-
Threshold frequencyLight of a frequency called the threshold frequency has photons of energy equal to the work function
-
when light photons shine on the metalLight photons shining on the metal can either give all of their energy or none of it.One photon will be absorbed by one electron.If the photon’s energy is equal to the work function of the metal the electron will escape.
-
What happens if you shine light of a high enough frequency onto the surface of the metalThe free electrons on the surface of the metal absorb energy from the light If an electron abdorbs enough energy, the bonds holding it to the metal break and the electron is released This is called the photoelectric effect and the electrons emitted are called photoelectrons
-
What is the groud state?the lowest energy level is called the ground state.
-
What happens to an electron if it gains alot of energyThe only place it CAN EXIST with more energy is a higher-energy orbit.
-
how to excite electronsHit them with an energetic external electronHit them with a photonBut there is a HUGE difference ...The orbital electron will absorb some or all of the external electron’s KEinelastic collision: yr 12 mechanicsThe orbital electron will absorb exactly all of the photon’s energyit is an all or nothing process!just like the photoelectric effect.
-
what will an electron in a high energy state do if there is space in a lower energy shellAn electron in a high energy state will spontaneously transition to a lower one, if there's space down there It's energy weill therefore decrease But that energy is not destroyed and gone from the universe forever A photon is emitted carrtying away the energy difference
-
equation for the photon emitted in deexcitation

-
How can electrons only exist in an atom?discrete energy levels
-
equation for the no. of photons (n) each secondE(TOTAL)=nhf
-
Hertz experimentWhen two beams of radio waves meet they produce interference and was able to see a tiny spark in the receiverThis spark was more vigorous when exposed to UV light from the transmitter sparkHertz measured the interference pattern that two radio waves produced to find their speedHe discovered that they moved at the same speed as light
-
work funciton equationThreshold frequency : hfmin = 𝜙Different for different metals
-
what is stopping potentialThe minimum potential needed to stop photoelectric emission
-
what happens at stopping potentialAt this potential, the maximum kinetic energy of the emitted electron is reduced to zero because each emitted electron must do extra work to equal to e(electron charge) x Vs to leave the surface
-
Photoelectric effect according to the photon modelWhen light hits its surface, the metal is bombarded by photons If one of these photons collides with a free electron, the electron will gain energy equal to hf
-
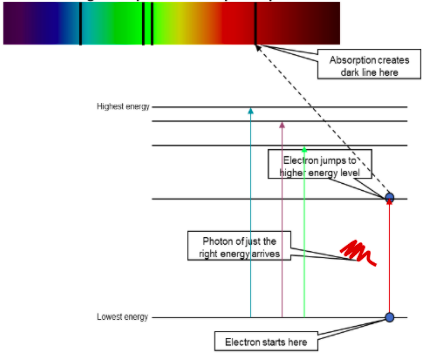
What happens when light is shone through elements (emission spectrum)Light shining through elements might be absorbed by them. If only certain elements are present, only certain frequencies will be absorbed (ΔE=hf) The pattern of dark lines is a “fingerprint” for each element.
-
Absorption spectrum diagram and explaination
-
why do gasses have a unique emission spectrum?each gas can only emit certain photons as electrons inside atoms can only have fixed levels of energy, i.e. they are quantasised. The discrete energyy levels (i.e. the allowable energy) is unique to each element
-
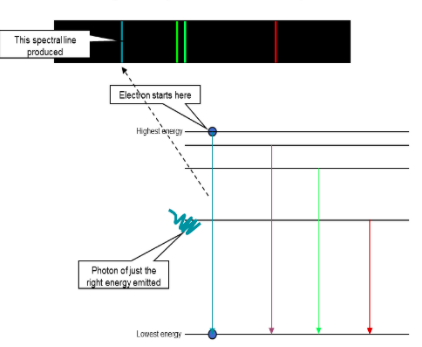
Emission spectrum diagram
-
emission lines vs absorption linesemission lines refer to the fact that glowing hot gas emits lines of light absorption lines refer to the tendency of cool gas to absorb the same lines of light
-
How does the line absorption spectrum occurr?When white light is passed through a cool gas At low temperatures, most of the electrons in the gas atoms will be in their ground states The electrons can only absorb photons with energies equal to the difference between two energy levels Photons of the corresponding wavelengths are absorbed by the electrons to excite them to higher energy levels These wavelengths are then missig from the continuous spectrum with black lines
-
Why are phtons absorbed and emitted have certain energiesThe energy of each photon emitted can only take a certain value as these transitions are between discrete energy levels
-
Hertz experiment conclusionShowed that waves Travelled in straight linesCould be reflected from a metal mirrorCould be polarisedCould refract through a prism
-
Experimental findings from when UV light is shown on negatively charged gold leaf experimentElectrons are only emitted when light of a high enough frequency is used. Below this frequency no electrons are emitted, no matter how bright the light. Electrons are emitted instantly or not at allKinetic energy of electrons depends on the frequency of the lightIncreasing the intensity of the light increases the number of electrons emitted, not their kinetic energies.
-
Explaination for UV light is shone on negatively charged gold leaf experimentA (UV) photon hits an electron at the surface of a metal.It, and its energy, is completely absorbed by the electron - this is an all-or-nothing process.If the electron receives enough KE, it is ejected from the metal - becoming a photoelectron.
-
intensity dependencyChanges the rate of photoemissionBut only if the frequency is above threshold
-
what happens if the photons has a frequency even greater than the threshold frequencyIf the photons has a frequency even greater than the threshold frequency, the electrons will also have kinetic energy as they escape
-
Why cant the photoelectric effect be explained by wave theoryFor a particular frequency of light, the energy carried is proportional to the intensity of the beam The energy carried by the light would be spread evenly over the wavefront Each free electron on the surace of the metal would gain a bit of energy from each incoming wave Gradually each electron would gain enough energy to leave the metal SO... The higher the intensity of the wave, the more energy it should transfer to each electron — the kinetic energy should increase with intensity. There’s no explanation for the kinetic energy depending only on the frequency.There is also no explanation for the threshold frequency. According to wave theory, the electrons should be emitted eventually, no matter what the frequency is.
-
How does the photon model explain threshold frequency?If the energy gained by an electron (on the surface of the metal) from a photon is greater than the work function, the electron is emitted If it isnt the metal will heat up but no electons are emitted
-
How does the photon model explain maximum kinetic energyThe energy transferred to the electron is hf The kinetic the electro will be carrying when it leaves the metal is hf minus any energy its lost on the way out. Electrons deeper down in the metal lose more energy than the electrons on th surface, which explains therange of energies The minimum ammount of energy it can lose is the work function The kinetic energy of the electrons is independent of the intensity as they can only absorb one photon at a time. Increasing the intensity only increases the photons per second on the area but each photon has the same energy as before
-
stopping potentialThe maximum kinetic energy can be measured using the idea of stopping potential The emitted electrons are made to lose their energy by doing the work done agaist an applied potential difference The stopping potential is the potential difference needed to stop fast moving electrons with max ke The work done by the poential difference in stopping the fastest electrons is equal to the energy they were carrying
-
stopping potential equation

-
How can electrons transition from one state to another?Electrons may transition from one state to another, gaining or losing energy.‘Upward’ transitions require a source of energy:the electron has to be given some energy somehow,these transitions are called excitations.
-
What happens after an electron excites?The electron will‘fall back down’ into one of the lower levelsand emit some light (flouresence)
-
Ionisation?The ionisation energy of an atom is the minimum energy required to remove an electron from an atom, and ionise the atom.As before:A sufficiently energetic photonall-or-nothing processA fast-moving external electronsome or all of the KE is usedinelastic collision
-
The states get closer together as...?they get closer to the ionisation energies
-
Forms of deexcitation?Deexcitation can be a cascade wherein several lower energy photons are emitted or a single event wherein one high-energy photon is emitted
-
What happens when gas is heated?Gas is heated, electrons move from a stable configuration of energy levels into an unstable one. But they cannot remain in an excited state for long, the electrons move back to the lower energy levels, emitting photons.
-
in de-excitation energy of the emitted photon

-
What happens when objects are hot?Hot things emit a continuous spectrum in the visible and infared All the wavelengths are allowed because the electrons are not confined to energy levels in the object producing the continuous spectrum. The electrons are not bound to atoms and are free
-
how does a discharge tube work?Apply a high voltage across a low-pressure gas When a current flows, electrons are accelerated by the potential difference They collide with gas atoms and excite them The result is a comple emission spectrum, with some lines in the same place as the absorption spectrum
-
what is the energy gained by an electron equal to (eV)?accelerating voltage (V)?
-
How does the cathode ray experiment suggest electrons behave like particles
Electrons carry momentum and excite other electrons in atoms
Can carry charge and can be accelerated in an electric field
-
How the pattern in an electron diffraction tube supports the idea that the electron beam is acting as a wave than a stream of particles
Particles would scatter randomly
Wave property shown by diffraction
Maximum intensity occurs when the waves interfere constructively
-
How emission of light from the fluorescent screen in an electron diffraction tube shows that the electrons incident on it are behaving as particles
The atoms interact and transfer energy in a one to one interaction
Collisions by incident electrons move electrons in atoms to in between energy levels
Light emitted due to the excitation n deexcitation of electrons
-
Maximum kinetic energy
hf - work function

