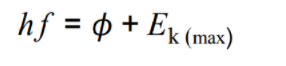



How does the cathode ray experiment suggest electrons behave like particles
Electrons carry momentum and excite other electrons in atoms
Can carry charge and can be accelerated in an electric field
How the pattern in an electron diffraction tube supports the idea that the electron beam is acting as a wave than a stream of particles
Particles would scatter randomly
Wave property shown by diffraction
Maximum intensity occurs when the waves interfere constructively
How emission of light from the fluorescent screen in an electron diffraction tube shows that the electrons incident on it are behaving as particles
The atoms interact and transfer energy in a one to one interaction
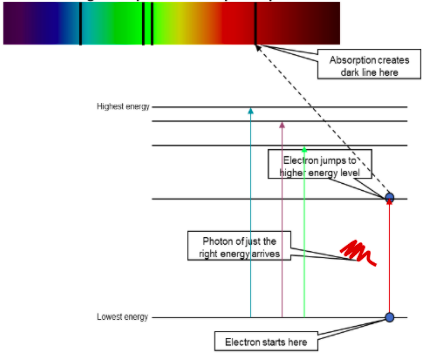
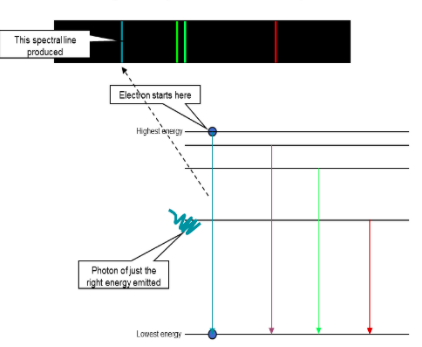
Collisions by incident electrons move electrons in atoms to in between energy levels
Light emitted due to the excitation n deexcitation of electrons
Maximum kinetic energy
hf - work function