-
Oxyacids
Acid H+. attached to oxygen
ex; Sulfuric Acid (H2SO4), Nitric Acid (HNO3)
-
Carboxylic Acid
Acid + COOH
;Citric Acid (H3C6H5O7)
-
What color does a base turn red litmus?
Red -> Blue
-
NaOH
Base; Sodium Hydroxide
-
KOH
Base;Potassium Hydroxide
-
Sodium Bicarbonate
Base; NaHCO3
-
Sodium Carbonate
Na2CO3; Base
-
AMMONIA
NH3; Base
-
what color is acid in Phenolphthalein?
clear
-
What color is phenolphthalein with base?
pink
-
Define a Bonsted-Lowry Acid
Element that donates H+
-
Define Bronsted-Lowry Base
Element that accepts H+ from donater
-
What is a conjugate base?
the elemental residue from the acid that donated its H+
-
What is a conjugate acid?
The element that accepts and integrates the H+ donation
-
What is an acid in arrhenius theory?
A substance that releases H+
-
What is a base in arrhenius theory?
a substance that produces oh- ions
-
What is the percent of ionization of strong acids?
100% ionization
-
HCL
Strong Acid; Hydrochloric Acid
-
Hydrobromic Acid
Strong Acid; HBr
-
Hydriodic Acid
Strong Acid; HI
-
Nitric Acid
Strong Acid; HNO3
-
Perchloric Acid
Strong acid; HCLO4
-
Sulfuric Acid
H2SO4; Strong Acid
-
HF
Hydrofluoric Acid; Weak acid
-
Acetic Acid
HC2H3O2; Weak Acid
-
Formic Acid
HCHO2; Weak Acid
-
Sulfurous Acid
H2SO3; Weak Acid
-
Carbonic Acid
Weak Acid; H2CO3
-
Phosphoric Acid
Weak Acid; H3PO4
-
What is an amphoteric substance; provide example
A substance that can be an acid or a base; Water
-
What is the ion product of water; or Dissociation constant?
Kw; 1.00 x 10^-14 @ 25C
-
What is the formula for the Acid Ionization Constant?
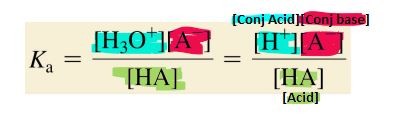
-
What is the formula for pH?

-
What is the formula for pOH?
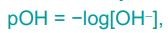
-
What is pH + pOH= ?
14
-
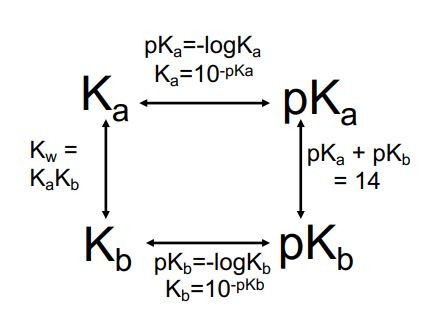
Draw the Acid/Base square