Acid-base balance (Physiology)
Session Summary In this session you will learn about how hydrogen ions are produced as a by-product of ordinary metabolism, and the various methods we have evolved to deal with this. Our blood contains potent buffering mechanisms, and our kidneys (supported by the removal of CO2 by the lungs) continually act to remove unwanted acids while recycling our bodies' buffering molecules. The role of the kidney in maintaining acid-base balance is critical for normal bodily health. The material in this session was adapted from a previous lecture written by Dr Suman Rice. Learning outcomes At the end of this session you will be able to: Understand where hydrogen ions come from & why controlling pH is important Understand pH buffering in the plasma Describe the secretion of hydrogen ions and the “reabsorption” of bicarbonate ions in the renal tubule Name other buffers present in tubule, e.g. phosphate and ammonia Understand the links between blood, lung and kidneys in acid-base balance Session resources Download the lecture slides: Acid-Base Balance 2023-2024 Berwick.pptxDownload Acid-Base Balance 2023-2024 Berwick.pptx Session activities Introduction As you should remember from High School, the pH of a liquid is the -log10 its hydrogen ion concentration. This means the normal pH range of bodily fluids, which is 7.35-7.45, corresponds to a hydrogen ion concentration of ≈0.00000004 moles per litre, or 40 nM. It is vital that this pH range is maintained. You should also be well aware that the metabolism of carbohydrates and fats (i.e. our major energy sources) continually produces carbon dioxide as a waste product. Carbon dioxide is removed from the body via the lungs. What you may be less aware of is the demands this CO2 production places on the pH of our bodies. Carbon dioxide dissolves in the aqueous environment of the body to produce carbonic acid: CO2 + H2O ↔ H2CO3 Carbonic acid is a volatile acid that readily dissociates into hydrogen ions and carbonate ions: CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3- So our normal metabolism leads the continual production of hydrogen ions! face-screaming-in-fear_1f631.png Complicating this further, our cellular metabolism produces other acid compounds that in many cases are fixed acids (i.e. not volatile acids) and even more dangerous. For example, the metabolism of the sulfur-containing amino acids methionine and cysteine creates sulphuric acid, whereas the metabolism of the basic amino acids lysine, arginine and histidine leads to the production of hydrochloric acid. Again, face-screaming-in-fear_1f631.png But fortunately human being do not simply dissolve in a puddle of their own metabolic waste. The body employs three complementary tactics to maintain pH. As depicted in the image below, these are: buffering (of intracellular and extracellular fluids); the respiratory system; and the kidney. These processes are the subject of this lecture.
-
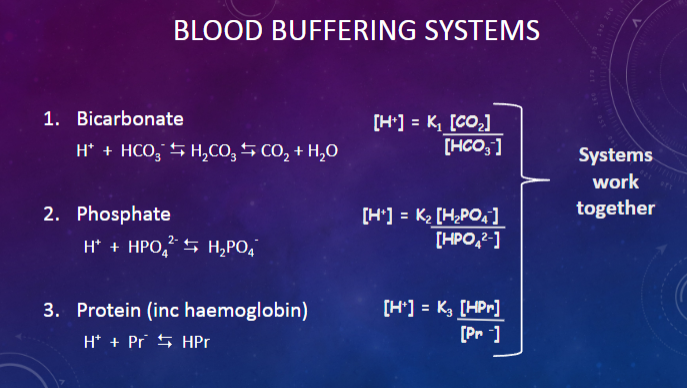
Picture demonstrating blood buffering systems and how they work together:
-
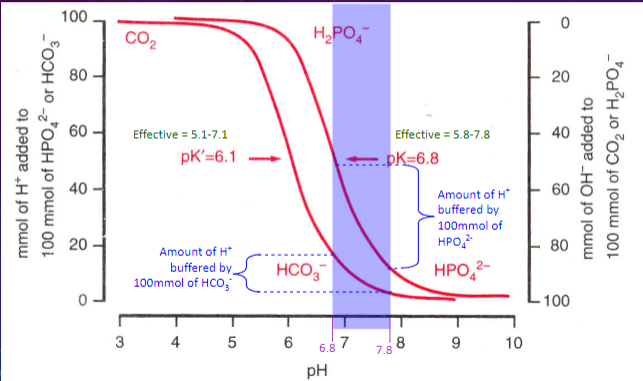
Picture demonstrating pH buffering:
-
What are the blood buffering systems? (6)
Bicarbonate System:
✿H++HCO3−⇌H2CO3⇌CO2+H2OH++HCO3−⇌H2CO3⇌CO2+H2O
Phosphate System:
✿H++HPO42−⇌H2PO4−H++HPO42−⇌H2PO4−
Protein System (including hemoglobin):
✿H++Pr−⇌HPrH++Pr−⇌HPr
✿[Pr−]=K3([HPr][HPO42−])[Pr−]=K3([HPO42−][HPr])
✿[H+]=K2([H2PO4−][HCO3−])[H+]=K2([HCO3−][H2PO4−])
✿[H+]=K1([CO2][HCO3−])[H+]=K1([HCO3−][CO2])
-
What is the Henderson-Hasselbalch equation used for in the context of the bicarbonate buffer system? (6)
✿The Henderson-Hasselbalch equation is used to calculate the pH of a solution based on the concentrations of bicarbonate ion (HCO3−) and carbon dioxide (CO2), as well as the dissociation constant (K1).
✿The equation is: pH=pK+log([HCO3−][CO2])pH=pK+log([CO2][HCO3−])
✿In clinical practice, the bicarbonate to carbon dioxide ratio ([HCO3−][CO2][CO2][HCO3−]) is typically measured to assess acid-base balance in the blood.
✿The ratio is determined using the concentrations of bicarbonate (HCO3−HCO3−) and the partial pressure of carbon dioxide (pCO2pCO2) in arterial blood gases.
✿The conversion factor to relate pCO2 (in mmHg) to CO2 concentration (in mmol/L) is approximately 0.03.
✿The Henderson-Hasselbalch equation provides a way to estimate pH based on these measured values.
-
What are the pros (4) and cons (2) of bicarbonate buffering?
✿Pro: Independent regulation
✿Pro: Abundant source of CO2 from metabolism
✿Pro: Alveolar ventilation controls PCO2
✿Pro: Kidneys control extracellular fluid bicarbonate concentration
✿Con: At pH 6.1, the pK of the CO2-HCO3 buffer is not close to the desired plasma pH of 7.4
✿Con: From a purely chemical point of view, it is not an ideal choice as a buffer solution.
-
What are the primary renal mechanisms involved in the control of acid-base levels? (6)
✿"Re-absorption" and secretion of HCO3
✿Formation of "new" HCO3
✿Secretion of [H+] into tubular fluid
Buffer systems within the tubule that react with secreted [H+] are:
✿HCO3 : H2CO3
✿HPO42 -: H2PO4
✿NH3: NH4+
-
Picture demonstrating the kidneys and the buffering system:
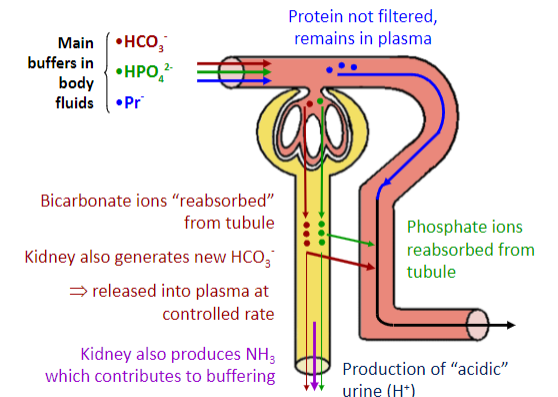
-
Picture demonstrating the renal control of [H+] & [HCO3-]:
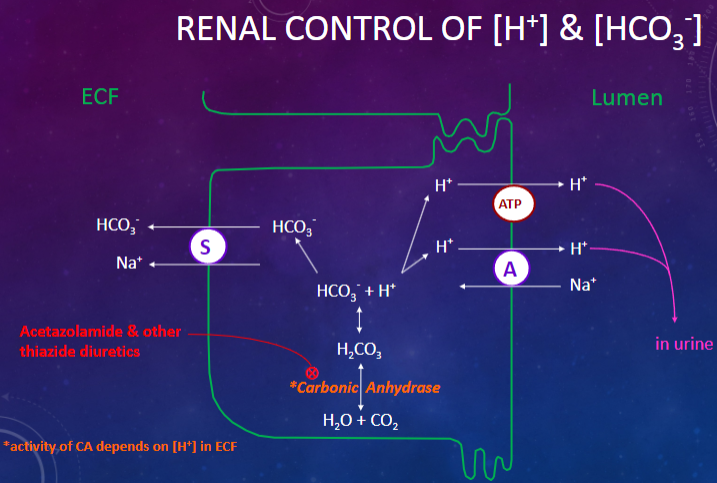
-
Picture demonstrating reabsorption in the proximal tubule:
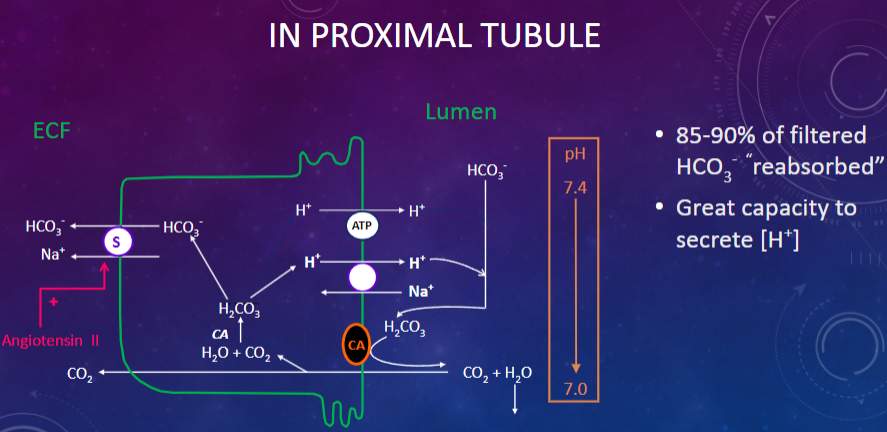
-
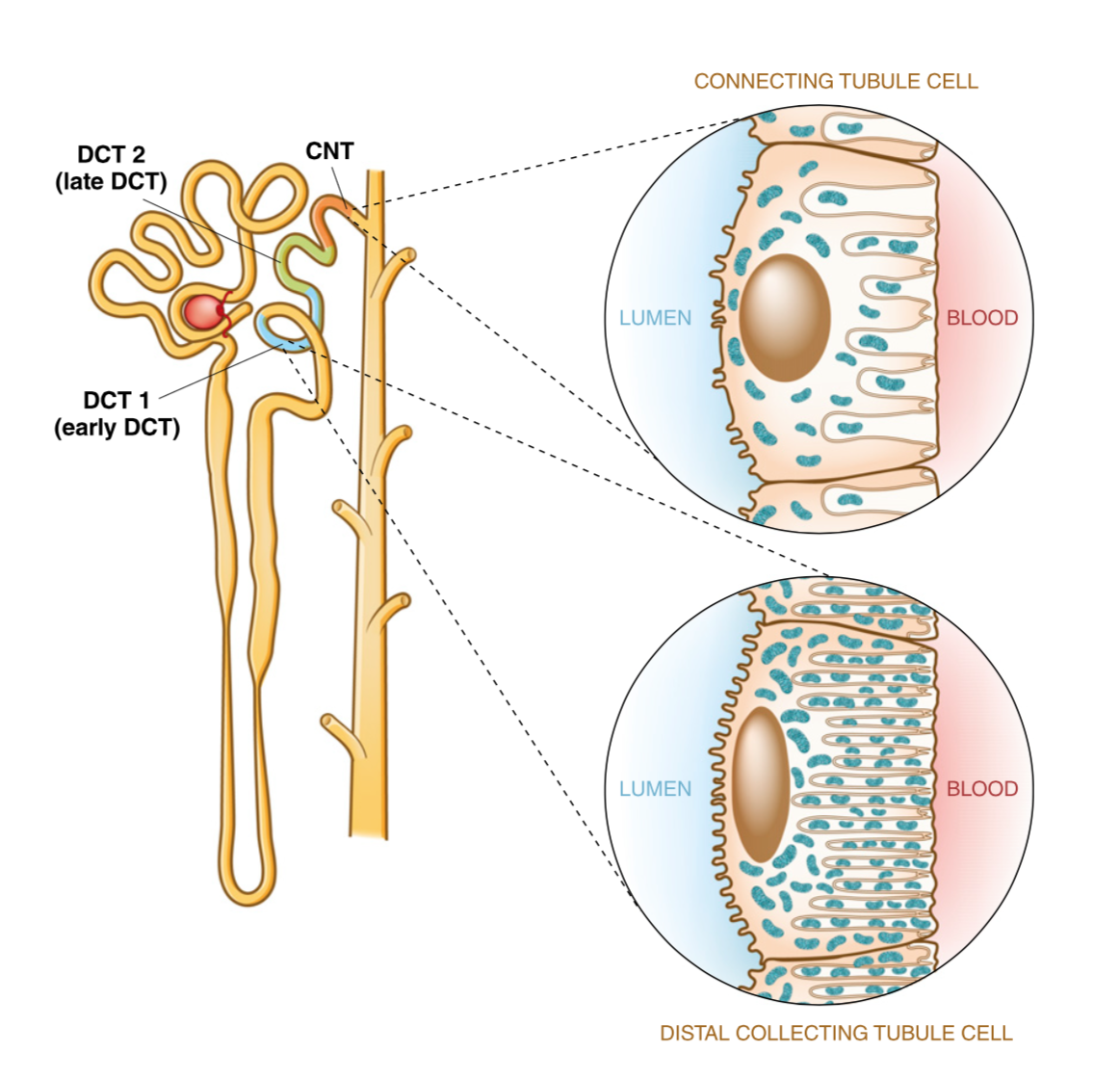
What is the primary function of intercalated cells in the late distal convoluted tubule and collecting duct? (2)
![✿In distal part of nephron [HCO3 ] is low and H+ react with other buffers✿H+-ATPase pump most important because it facilitates the secretion of protons/H+ into the tubular fluid](/flashcards/cardimage2/54d373b3/269/8269933_back.png)
✿In distal part of nephron [HCO3 ] is low and H+ react with other buffers
✿H+-ATPase pump most important because it facilitates the secretion of protons/H+ into the tubular fluid
-
What is the role of phosphate as a buffer in the renal tubules? (2)
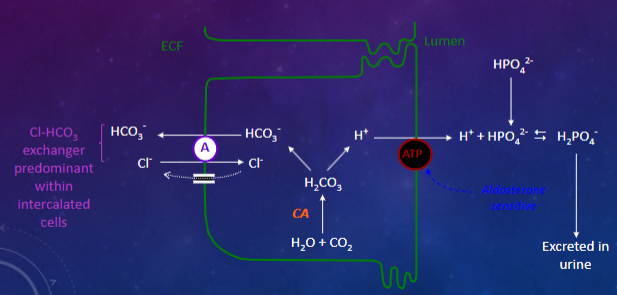
✿Further H+ secreted into lumen buffered by HPO42-
✿Very effective buffer because pK=6.8 (close to pH of filtrate)
-
What is the process by which tubular epithelium produces ammonia, and what is its significance in the context of metabolic acidosis? (4)
✿Tubular epithelium produces NH3 from glutamine with the enzyme glutaminase.
✿Excretion of ammonia salts increases tenfold during metabolic acidosis – from 30-50mmol/day to 300-500mmol/day.
✿One glutamine molecule gives rise to 2HCO3-.
✿Urinary excretion of ammonium salts. One glutamine molecule gives rise to 2HCO3-.
-
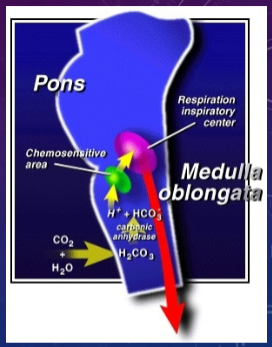
What is the role of the chemosensitive area in the medulla oblongata in regulating respiration? (3)
![✿The chemosensitive area in the medulla oblongata regulates respiration.✿It monitors [H+] of plasma indirectly, via CSF (Cerebral Spinal Fluid).✿Charged ions cannot cross the BBB (Blood Brain Barrier), but CO2 can.](/flashcards/cardimage2/230739bf/269/8269936_back.png)
✿The chemosensitive area in the medulla oblongata regulates respiration.
✿It monitors [H+] of plasma indirectly, via CSF (Cerebral Spinal Fluid).
✿Charged ions cannot cross the BBB (Blood Brain Barrier), but CO2 can.
-
What characterizes metabolic acidosis? (3)
![✿Metabolic acidosis is characterized by a low pH as a result of an increased extracellular fluid (ECF) [H+] or decreased ECF [HCO3-].✿It can be caused by severe sepsis or shock leading to lactic acid accumulation, uncontrolled diabetes resulting in the overproduction of 3-OH-butyric acid and other ketoacids, or diarrhea causing the loss of HCO3- from the gastrointestinal tract.✿The pH in metabolic acidosis can be calculated using the Henderson-Hasselbalch equation: pH = pK + log [HCO3-] / [CO2] x [HCO3-].](/flashcards/cardimage2/46774dfc/269/8269937_back.png)
✿Metabolic acidosis is characterized by a low pH as a result of an increased extracellular fluid (ECF) [H+] or decreased ECF [HCO3-].
✿It can be caused by severe sepsis or shock leading to lactic acid accumulation, uncontrolled diabetes resulting in the overproduction of 3-OH-butyric acid and other ketoacids, or diarrhea causing the loss of HCO3- from the gastrointestinal tract.
✿The pH in metabolic acidosis can be calculated using the Henderson-Hasselbalch equation: pH = pK + log [HCO3-] / [CO2] x [HCO3-].
-
Picture integrated renal & pulmonary compensation Acidosis:
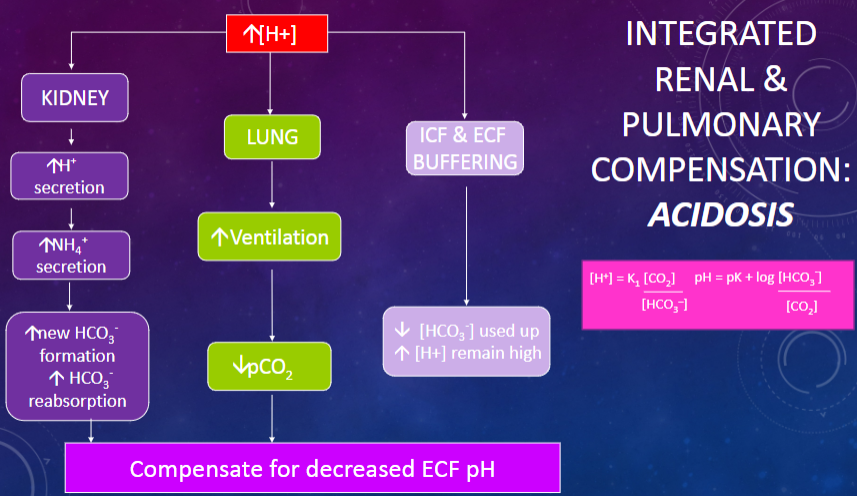
-
What characterizes metabolic alkalosis? (3)
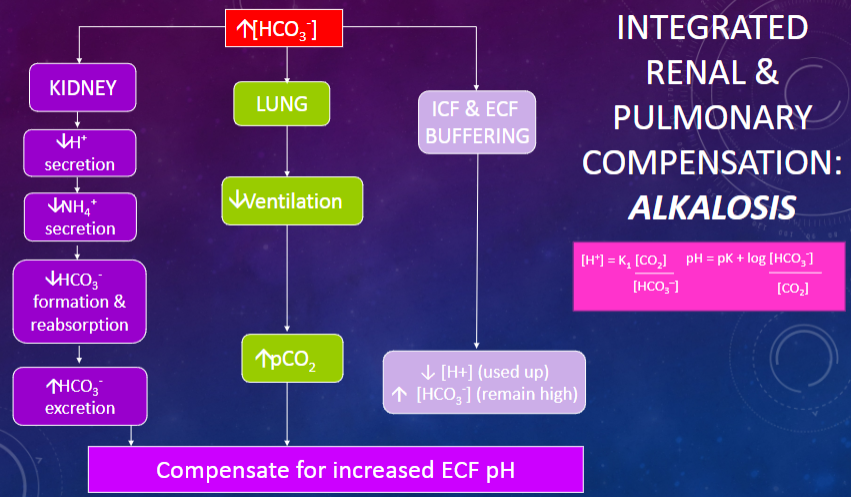
✿Metabolic alkalosis is characterized by a high pH resulting from an increased extracellular fluid (ECF) [HCO3-] or decreased ECF [H+].
✿It can be caused by excessive diuretic (thiazide) use leading to chronic loss of Cl-, Na+ & K+ and subsequent increase in H+ secretion, vomiting resulting in the loss of H+ from the gastrointestinal tract, ingestion of alkaline antacids, or hypokalemia.
✿The pH in metabolic alkalosis can be calculated using the Henderson-Hasselbalch equation: pH = pK + log [HCO3-] / [CO2] x [HCO3-].
-
Picture integrated renal & pulmonary compensation Alkalosis: