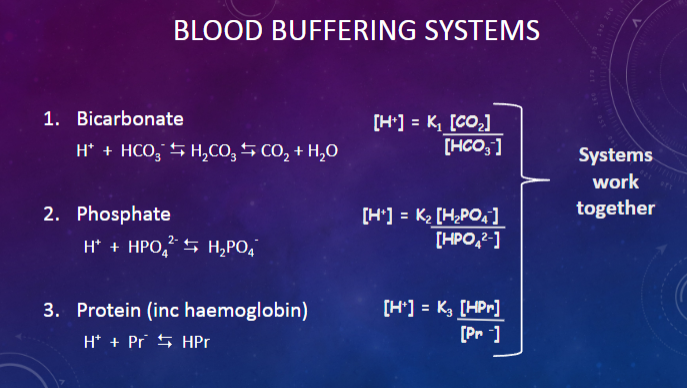
Picture demonstrating blood buffering systems and how they work together:
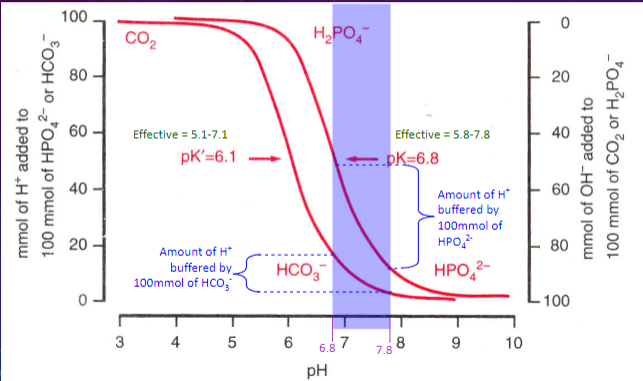
Picture demonstrating pH buffering:
What are the blood buffering systems? (6)
Bicarbonate System:
✿H++HCO3−⇌H2CO3⇌CO2+H2OH++HCO3−⇌H2CO3⇌CO2+H2O
Phosphate System:
✿H++HPO42−⇌H2PO4−H++HPO42−⇌H2PO4−
Protein System (including hemoglobin):
✿H++Pr−⇌HPrH++Pr−⇌HPr
✿[Pr−]=K3([HPr][HPO42−])[Pr−]=K3([HPO42−][HPr])
✿[H+]=K2([H2PO4−][HCO3−])[H+]=K2([HCO3−][H2PO4−])
✿[H+]=K1([CO2][HCO3−])[H+]=K1([HCO3−][CO2])
What is the Henderson-Hasselbalch equation used for in the context of the bicarbonate buffer system? (6)
✿The Henderson-Hasselbalch equation is used to calculate the pH of a solution based on the concentrations of bicarbonate ion (HCO3−) and carbon dioxide (CO2), as well as the dissociation constant (K1).
✿The equation is: pH=pK+log([HCO3−][CO2])pH=pK+log([CO2][HCO3−])
✿In clinical practice, the bicarbonate to carbon dioxide ratio ([HCO3−][CO2][CO2][HCO3−]) is typically measured to assess acid-base balance in the blood.
✿The ratio is determined using the concentrations of bicarbonate (HCO3−HCO3−) and the partial pressure of carbon dioxide (pCO2pCO2) in arterial blood gases.
✿The conversion factor to relate pCO2 (in mmHg) to CO2 concentration (in mmol/L) is approximately 0.03.
✿The Henderson-Hasselbalch equation provides a way to estimate pH based on these measured values.
What are the pros (4) and cons (2) of bicarbonate buffering?
✿Pro: Independent regulation
✿Pro: Abundant source of CO2 from metabolism
✿Pro: Alveolar ventilation controls PCO2
✿Pro: Kidneys control extracellular fluid bicarbonate concentration
✿Con: At pH 6.1, the pK of the CO2-HCO3 buffer is not close to the desired plasma pH of 7.4
✿Con: From a purely chemical point of view, it is not an ideal choice as a buffer solution.
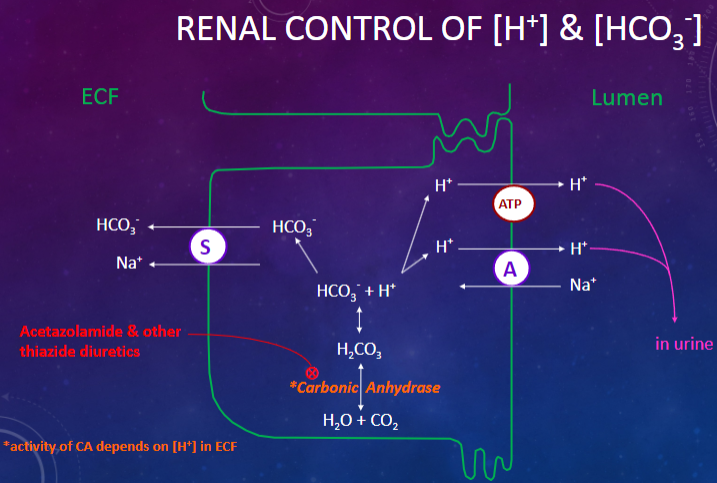
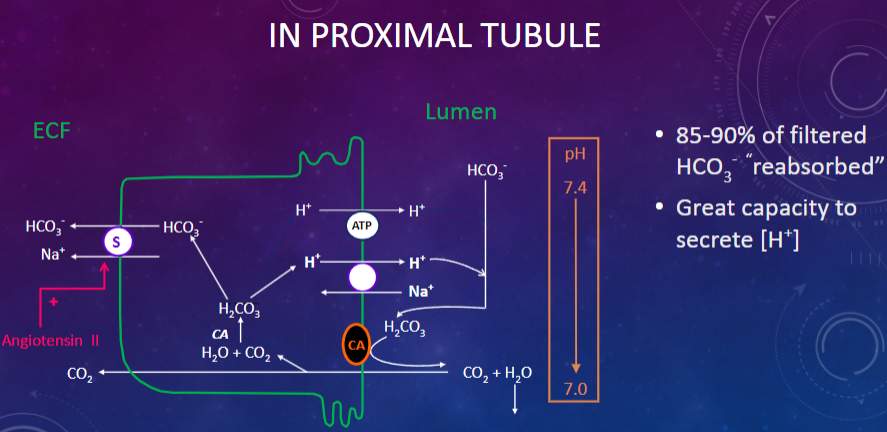
What are the primary renal mechanisms involved in the control of acid-base levels? (6)
✿"Re-absorption" and secretion of HCO3
✿Formation of "new" HCO3
✿Secretion of [H+] into tubular fluid
Buffer systems within the tubule that react with secreted [H+] are:
✿HCO3 : H2CO3
✿HPO42 -: H2PO4
✿NH3: NH4+
Picture demonstrating the kidneys and the buffering system:
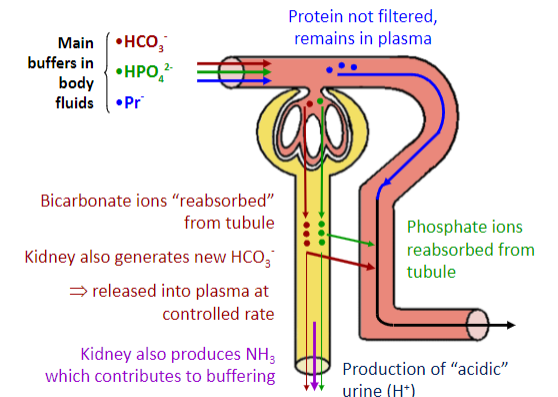
Picture demonstrating the renal control of [H+] & [HCO3-]:
Picture demonstrating reabsorption in the proximal tubule:
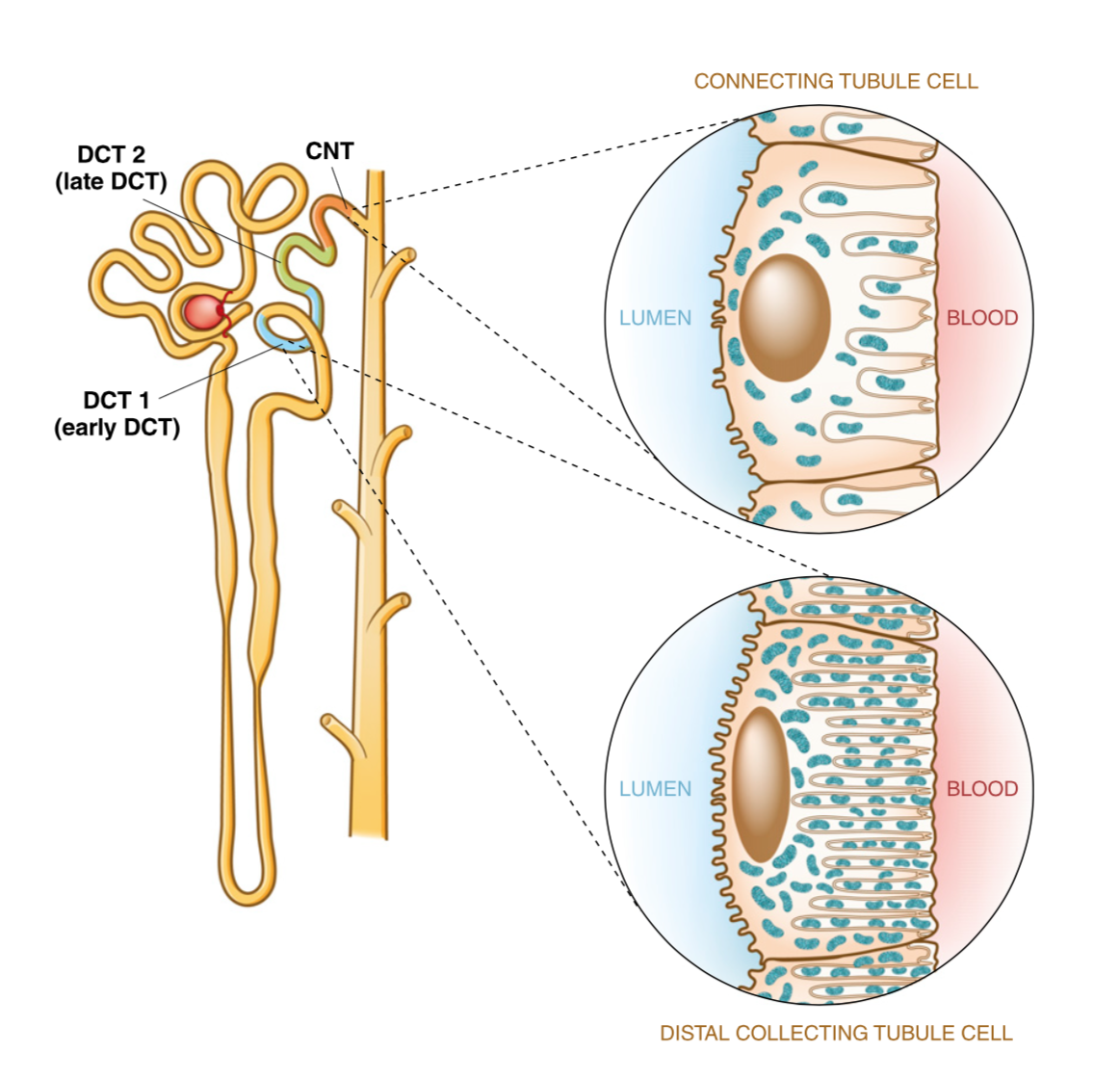
What is the primary function of intercalated cells in the late distal convoluted tubule and collecting duct? (2)
![<p>✿In distal part of nephron [HCO3 ] is low and H+ react with other buffers</p><p>✿H+-ATPase pump most important because it facilitates the secretion of protons/H+ into the tubular fluid</p>](/flashcards/cardimage2/54d373b3/269/8269933_back.png)
✿In distal part of nephron [HCO3 ] is low and H+ react with other buffers
✿H+-ATPase pump most important because it facilitates the secretion of protons/H+ into the tubular fluid
What is the role of phosphate as a buffer in the renal tubules? (2)
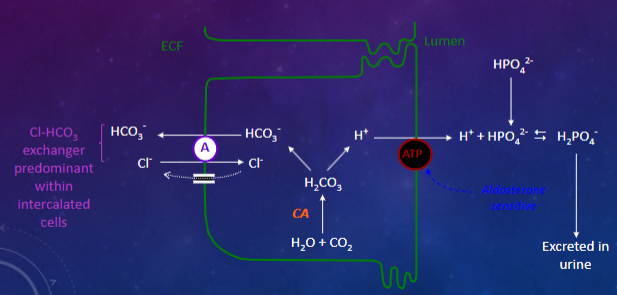
✿Further H+ secreted into lumen buffered by HPO42-
✿Very effective buffer because pK=6.8 (close to pH of filtrate)
What is the process by which tubular epithelium produces ammonia, and what is its significance in the context of metabolic acidosis? (4)
✿Tubular epithelium produces NH3 from glutamine with the enzyme glutaminase.
✿Excretion of ammonia salts increases tenfold during metabolic acidosis – from 30-50mmol/day to 300-500mmol/day.
✿One glutamine molecule gives rise to 2HCO3-.
✿Urinary excretion of ammonium salts. One glutamine molecule gives rise to 2HCO3-.
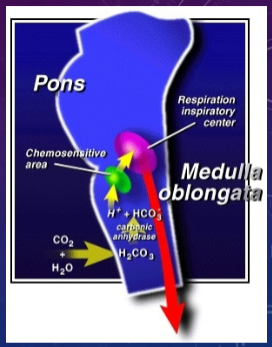
What is the role of the chemosensitive area in the medulla oblongata in regulating respiration? (3)
![<p>✿The chemosensitive area in the medulla oblongata <strong>regulates respiration</strong>.</p><p>✿It monitors [H+] of plasma indirectly, via CSF (Cerebral Spinal Fluid).</p><p>✿Charged ions cannot cross the BBB (Blood Brain Barrier), but CO2 can.</p>](/flashcards/cardimage2/230739bf/269/8269936_back.png)
✿The chemosensitive area in the medulla oblongata regulates respiration.
✿It monitors [H+] of plasma indirectly, via CSF (Cerebral Spinal Fluid).
✿Charged ions cannot cross the BBB (Blood Brain Barrier), but CO2 can.
What characterizes metabolic acidosis? (3)
![<p>✿Metabolic acidosis is characterized by a low pH as a result of an increased extracellular fluid (ECF) [H+] or decreased ECF [HCO3-].</p><p>✿<strong>It can be caused by severe sepsis</strong> <strong>or shock</strong> leading to lactic acid accumulation, <strong>uncontrolled diabetes</strong> resulting in the overproduction of 3-OH-butyric acid and other ketoacids, <strong>or diarrhea</strong> causing the loss of HCO3- from the gastrointestinal tract.</p><p>✿The pH in metabolic acidosis can be calculated using the Henderson-Hasselbalch equation: pH = pK + log [HCO3-] / [CO2] x [HCO3-].</p>](/flashcards/cardimage2/46774dfc/269/8269937_back.png)
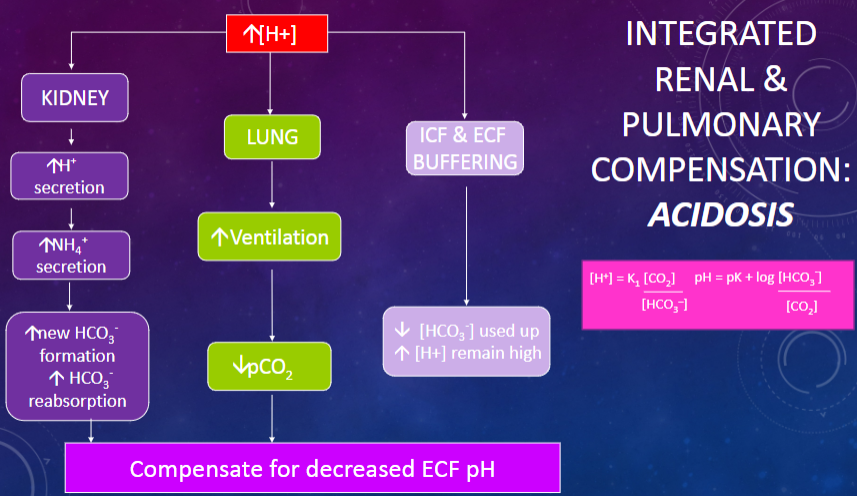
✿Metabolic acidosis is characterized by a low pH as a result of an increased extracellular fluid (ECF) [H+] or decreased ECF [HCO3-].
✿It can be caused by severe sepsis or shock leading to lactic acid accumulation, uncontrolled diabetes resulting in the overproduction of 3-OH-butyric acid and other ketoacids, or diarrhea causing the loss of HCO3- from the gastrointestinal tract.
✿The pH in metabolic acidosis can be calculated using the Henderson-Hasselbalch equation: pH = pK + log [HCO3-] / [CO2] x [HCO3-].
Picture integrated renal & pulmonary compensation Acidosis:
What characterizes metabolic alkalosis? (3)
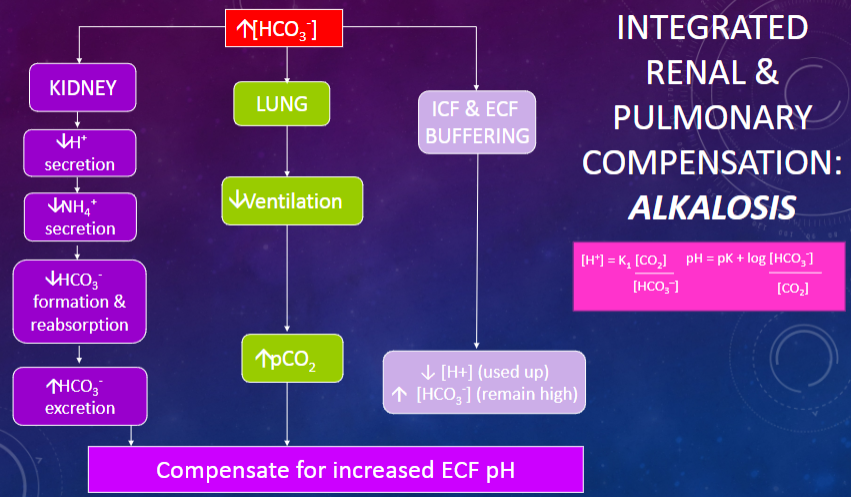
✿Metabolic alkalosis is characterized by a high pH resulting from an increased extracellular fluid (ECF) [HCO3-] or decreased ECF [H+].
✿It can be caused by excessive diuretic (thiazide) use leading to chronic loss of Cl-, Na+ & K+ and subsequent increase in H+ secretion, vomiting resulting in the loss of H+ from the gastrointestinal tract, ingestion of alkaline antacids, or hypokalemia.
✿The pH in metabolic alkalosis can be calculated using the Henderson-Hasselbalch equation: pH = pK + log [HCO3-] / [CO2] x [HCO3-].
Picture integrated renal & pulmonary compensation Alkalosis: