Chapter 4

Chapter 4
Types of Chemical Reactions and Solution Stoichiometry
Parts of solutions
Solution – homogeneous mixture
Solute – part that dissolves
Solvent – causes the dissolving
Soluble – can be dissolved
Miscible – liquids dissolve in each other
Saturation of Solutions
Aqueous Solutions
Dissolved in water
Water is a polar molecule
The oxygen atoms have a partial negative charge
The hydrogen atoms have a partial positive charge
The angle is 105 o
Hydration
The process of breaking apart ions of a salt.
The “+” end of water attracts the anion
The “-” end of water attracts the cation
Solubility
The ability to dissolve in a given amount of water
Usually g/100mL
Varies greatly
Depends upon ion attraction
Will dissolve nonionic substances if they have polar bonds
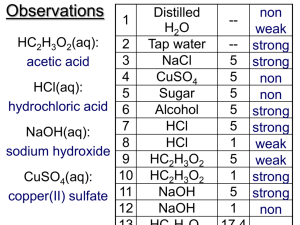
Electrolytes
Electrical current through a substance
Ions that are dissolved can move
Solutions are classified three ways
Types of Solutions
Strong electrolytes –Completely ionized when dissolved in water many ions – conduct well
Weak electrolytes – partially fall apart into ions few ions- conduct electricity slightly
Non-electrolytes – don’t fall apart no ions – don’t conduct electricity
Types of solutions continued
Acids – form H + ion when dissolved
Strong acids fall apart completely
H
2
SO
4
HNO
3
HCl HBr HI HClO
4
Weak acids – do not dissociate completely
Bases – form OH when dissolved
Strong bases – KOH NaOH
Dissociation
Acids
HCl H + (aq) + Cl (aq)
HNO
3
H + (aq) + NO
3
(aq)
H
2
SO
4
H + (aq) + HSO
4
(aq)
Strong bases
NaOH Na + (aq) + OH (aq)
KOH K + (aq) + OH (aq)
Nonelectrolytes
Dissolve in water but do not produce any ions
Example is ethanol (C
2
H
5
OH) molecules disperse in the water but doesn’t conduct electricity
Composition of Solutions
Concentration
1. Molarity (M) – moles of solute per volume of solution in liters
2. M = moles of solute liters of solution
Preparation of Molar Solutions
Calculate the molarity of a solution prepared by dissolving 1.56 g of gaseous HCl in enough water to make 26.8 mL of solution.
1.56 g HCl
1
1 mole HCl
36.5 g HCl
= 0.0427 mole HCl
26.8 mL
1
1L
1000 mL
= .0268 L
M = 0.0427 mole HCl
.0268 L
= 1.60 M HCl
Concentrations of Ions
Give the concentration of each type of ion in 0.50 M
Co(NO
3
)
2
Co(NO
3
)
2
(s) Co +2
(aq)
+ 2NO
3
-
(aq)
Co +2 1 x 0.50 M = 0.50 M Co +2
NO
3
2 x 0.50 M = 1.0 M NO
3
-
Calculate the number of moles of Cl -
1.75 L of 1.0 x 10 -3 M ZnCl
2
.
ions in
ZnCl
2
Zn +2
(aq)
+ 2Cl -
(aq)
2 x 1.0 x 10 -3 = 2.0 x 10 -3 M Cl -
1.75 L 2.0 x 10 -3 mole Cl -
L
= 3.5 x 10 -3 mole Cl -
Standard solution – a solution where the concentration is accurately known
How much solid K
2
Cr
2
O
7 must by weighted out to make a solution? A chemist needs 1.00 L of an aqueous 0.200 M K
2
Cr
2
O
7 solution.
Dilution
1. Water is added to achieve a particular M
2. Moles of solute after = moles of solute before
3.
M
1
V
1
=M
2
V
2
What volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M H
2
SO
4 solution?
Types of Solution Reactions
Precipitation Reactions
1. A solid forms from two solutions
2. Precipitate – the insoluble solid
KNO
3
(aq) + BaCl
2
(aq)
Three Types of Equations Used to Describe Reactions in a Solution
The formula equation gives the overall reaction.
The complete ionic equation represents as ions all the reactants and products that are strong electrolytes.
The net ionic equation includes only those ions that undergo a change.
Write the formula equation, complete ionic equation, and the net ionic equation for
KCl (aq) + AgNO
3
(aq)
Stoichiometry of Precipitation Reactions
Calculate the mass of solid NaCl that must be added to
1.50 L of a 0.100 M AgNO
3
Ag + ions in the form of AgCl.
solution to precipitate all the
When aqueous solutions of Na
2
SO
4 and Pb(NO
3
)
2 are mixed, PbSO
4
Pb(NO
3
)
2 formed when 1.25 L of 0.0500 M and 2.00 L of 0.0250 M Na
2
SO
4 are mixed.
Acid-Base Reactions
Acid is a proton donor (H + )
Base is a proton acceptor usually OH
— accepts a H +
H +
(aq)
+ OH
−
(aq)
Cations are surrounded and bound by water molecules protons are also solvated by water molecules
Two ways to show this
1.
H + (aq)
2.
H
3
O + (aq) – hydronium ion
Types of acid donors
Monoprotic
HCl, HNO
3 donates ____ H +
Diprotic
H
2
SO
4 donates ____ H +
Triprotic
H
3
PO
4 donates ____ H +
Neutralization Reactions
What volume of a 0.100 M HCl solution is needed to neutralize 25.0 mL of 0.350 M NaOH?
HCl
(aq)
+ NaOH
(aq)
NaCl
(aq)
+ H
2
O
(l)
H +(aq) + OH
−
(aq)
H
2
O
(l)
.0250 L 0.350 mole OH − =
L NaOH
Acid-Base Titrations
Volumetric analysis
Process of determining the amount of a substance by titration
Titration
Process of delivering solutions to one another
Equivalence point (stoichiometric point)
Point at which the titration has occurred
Endpoint
Point at which the indicator changes color
What volume of 0.812 M HCl, in milliliters, is required to titrate 1.33 g of NaOH to the equivalence point?
Oxidation-Reduction Reactions
Electrons are transferred
Spontaneous redox rxns can transfer energy
Electrons (electricity)
Heat
LEO the lion says GER
L
ose
E
lectrons =
O
xidation
G
ain
E
lectrons = eduction
Redox Reactions Examples:
0 0 +1
2Na + Cl
2
2NaCl
Each sodium atom oses one lectron
--1 oxidation
Each chlorine atom ains one lectron:
0
Cl + e __ Cl
-1 reduction
Rules for Assigning Oxidation
Numbers Rules 1 & 2
1. The oxidation number of any uncombined element is zero
2. The oxidation number of a monatomic ion equals its charge
3. The oxidation number of oxygen in compounds is -
2
4. The oxidation number of hydrogen in compounds is +1
5. The sum of the oxidation numbers in the formula of a compound is 0.
6. The sum of the oxidation numbers in the formula of a polyatomic ion is equal to its charge ex. NO
3
—
SO
4
2
—
Reducing Agents and Oxidizing Agents
The substance reduced is the oxidizing agent
The substance oxidized is the reducing agent
0 +1
Na Na + e --
Sodium is oxidized – it is the reducing agent
0 -- 1
Cl + e - Cl
Chlorine is reduced – it is the oxidizing agent
Oxidation-Number Changes in reactions
Can you identify what is being oxidized and what is being reduced?
2AgNO
3
(aq)
+ Cu
(s)
Cu(NO
3
)
2
(aq)
+ 2Ag
(s)
The oxidation number of Ag decreases from +1 to 0
(reduction), copper’s oxidation number increase from 0 to +2 (oxidation)
Balancing Redox Equations
Look at the reaction between solid copper and silver ions in aqueous solution:
Cu (s) + Ag + (aq) Ag (s) + Cu +2 (aq)
1 e -gained
Cu + Ag + Ag + Cu +2
2 e
lost
Ultimately must have equal number of electrons gained and lost
Cu (s) +