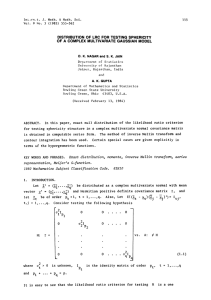
The pH Scale & pH Calculations
advertisement
The pH Scale & pH Calculations Chemistry 10 Mrs. Page Learning Objectives: • Explain and use the pH scale • Use a pH meter and universal indicators to determine the acidity or alkalinity of a substance • Solve problems involving pH and [H+] Explain and use the pH scale The pH Scale • pH is a measure of how acidic or basic a solution is, which means it is a measure of the concentration of hydrogen ions [H+] in a solution. • The pH scale ranges from 0 to 14. • Acidic solutions have pH values below 7 • A solution with a pH of 7 is neutral (pure water) • Basic/alkali solutions have pH values above 7. Explain and use the pH scale pH Scale • The pH scale is a way of expressing the amount of dissociation of acids and bases in water. • Instead of using very small numbers, we just use the NEGATIVE power of 10 on the Molarity of the H+ (or OH-) ions. Explain and use the pH scale pH Scale • The pH scale is a logarithmic scale (not linear). • A change of 1 pH unit represents a tenfold change in the acidity of the solution. • For example, if one solution has a pH of 1 and a second solution has a pH of 2, the first solution is not twice as acidic as the second—it is ten times more acidic. Measuring pH Use a pH meter and universal indicators to determine the acidity or alkalinity of a substance • Indicators are substances that turn different colors in the presence of an acid or base •Universal Indicator – color indicates pH •Litmus paper – turns red acid, turns blue base Measuring pH Use a pH meter and universal indicators to determine the acidity or alkalinity of a substance • Indicators are substances that turn different colors in the presence of an acid or base •Methyl orange: •Phenolphthalein: commonly used in commonly used in titrations, turns orange titrations, turns pink in in acidic solutions basic solutions Measuring pH • pH Sensor: digital tool for measuring pH • Must be used with a GoPro and Logger Pro software • Gives reading as a pH number Use a pH meter and universal indicators to determine the acidity or alkalinity of a substance pH Calculations Solve problems involving pH and [H+] pH = - log [H+] (Remember that the [ ] mean Molarity) Calculate the pH of a solution if the [H+] is 1 X 10-10 pH = -log [H+] = -log (1 x 10-10) pH = 10 CAREFUL: on you calculator use (-) for negative & use () around numbers pH Calculations Solve problems involving pH and [H+] pH = - log [H+] (Remember that the [ ] mean Molarity) Calculate the pH of a solution if the [H+] is 1.8 X 10-5 pH = -log [H+] = -log (1.8 x 10-5) pH = 4.7 pH Calculations Solve problems involving pH and [H+] pH = - log [H+] (Remember that the [ ] mean Molarity) Find the pH of a 0.15 M solution of Hydrochloric acid pH = -log [H+] = -log (0.15) pH = 0.82 pH Calculations Solve problems involving pH and [H+] pH = - log [H+] (Remember that the [ ] mean Molarity) Find the pH of a 3.00 X 10-7 M solution of Nitric acid pH = -log [H+] = -log (3.00 x 10-7) pH = 6.52 Solve problems involving pH and [H+] pH Calculations If you have pH you can also calculate the [H+] pH = - log [H+] -pH = log [H+] Take antilog (10x) of both sides and get -pH 10 = [H+] * to find antilog on your calculator, look for “Shift” or “2nd function” and then the log button Solve problems involving pH and [H+] pH Calculations If the pH of Coke is 3.12, what is the [H+] ? -pH 10 = [H+] -3.12 10 = [H+] [H+] = 7.59 x -4 10 M Solve problems involving pH and [H+] pH Calculations A solution has a pH of 8.5. What is the Molarity of hydrogen ions in the solution? -pH 10 = [H+] -13.4 10 = [H+] [H+] = 3.2 x -9 10 M pH Calculations Solve problems involving pH and [H+] A solution has a pH of 1.5. What is the Molarity of hydrogen ions in the solution? -pH 10 = [H+] -8.5 10 = [H+] [H+] = 0.032M