Energy
advertisement

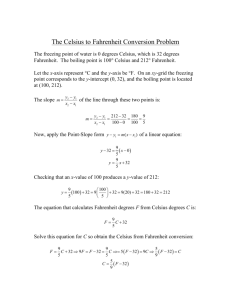
Energy is defined as the ability to do work. Work = force x distance The ability to do work. Work - cause a change or move an object. Many types- all can be changed into the other. Potential- stored energy ◦ Position, condition or composition Kinetic Energy- energy something has because its moving Heat- the energy that moves because of a temperature difference. Chemical energy- energy released or absorbed in a chemical change. Electrical energy - energy of moving charges Radiant Energy- energy that can travel through empty space (light, UV, infrared, radio) Nuclear Energy – Energy from changing the nucleus of atoms *All types of energy can be converted into others. If you trace the source far enough back, you will end up at nuclear energy. Energy can be neither created or destroyed in ordinary changes (not nuclear), it can only change form. Discovered by Julius Robert Mayer in 1842 Now called: The First Law of Thermodynamics Law of Conservation of Mass - Energy The total amount of mass and energy in the universe is constant. Some theories are based on supporting postulates. A postulate is a statement which is agreed on by consensus among scientists. The following are important postulates of the kinetic molecular theory: All matter consists of atoms. Atoms may join together to form molecules. Solids usually maintain both their shape and their volume. Liquids maintain their volume, but not their shape. Gases do not maintain shape or volume. They will expand to fill a container of any size. Molecular motion is random. Molecular motion is greatest in gases, less in liquids, and least in solids. Collisions between atoms and molecules transfers energy between them. Molecules in motion possess kinetic energy. Molecules in gases do not exert large forces on one another, unless they are colliding. Also see chapter 11 of textbook Thermal energy is the average of the potential and kinetic energies possessed by atoms and molecules experiencing random motion. Heat is transferred by convection, conduction, or radiation. (review the definitions of these words) Heat is the thermal energy transferred from one object to another due to differences in temperature. Heat flow from high to low temperature. There is no direct method used to measure heat. Indirect methods must be used. Temperature is a measure of the average kinetic energy of the molecules of a substance. There is a direct relationship between temperature and avg. kinetic energy! Temperature can be measured with a thermometer. One way a thermometer can be calibrated is by the amount of thermal expansion and contraction that occurs within a given type of substance. Thermometers are limited by the physical properties of the substance from which they are made. (i.e., An alcohol thermometer is of little use above the boiling point of alcohol, and a mercury thermometer will not be of any use below the freezing point of mercury.) Both scales are based on the freezing conditions of water, a very common and available liquid. Since water freezes and boils at temperatures that are rather easy to generate (even before modern refrigeration), it is the most likely substance on which to base a temperature scale. 100ºC = 212ºF 0ºC = 32ºF 0ºC 32ºF 100ºC 212ºF Zero Fahrenheit was the coldest temperature that the German-born scientist Gabriel Daniel Fahrenheit could create with a mixture of ice and ordinary salt. He invented the mercury thermometer and introduced it and his scale in 1714 in Holland, where he lived most of his life. Anders Celsius, a Swedish astronomer, introduced his scale is 1742. For it, he used the freezing point of water as zero and the boiling point as 100. For a long time, the Celsius scale was called "centigrade." The Greek prefix "centi" means one-hundredth and each degree Celsius is one-hundredth of the way between the temperatures of freezing and boiling for water. The Celsius temperature scale is part of the "metric system" of measurement (SI) and is used throughout the world, though not yet embraced by the American public. How much it changes 100ºC = 212ºF 0ºC = 32ºF 100ºC = 180ºF 0ºC 100ºC 212ºF 32ºF How much it changes 100ºC = 212ºF 0ºC = 32ºF 100ºC = 180ºF 1ºC = (180/100)ºF 1ºC = 9/5ºF 0ºC 100ºC 212ºF 32ºF Scientists use a third scale, called the "absolute" or Kelvin scale. This scale was invented by William Thomson, Lord Kelvin, a British scientist who made important discoveries about heat in the 1800's. Scientists have determined that the coldest it can get (theoretically) is minus 273.15 degrees Celsius. This temperature has never actually been reached, though scientists have come close. The value, minus 273.15 degrees Celsius, is called "absolute zero". At this temperature scientists believe that molecular motion would stop. You can't get any colder than that. The Kelvin scale uses this number as zero. To get other temperatures in the Kelvin scale, you add 273 degrees to the Celsius temperature. The important idea is that temperature is really a measure of something, the average motion (kinetic energy, KE) of the molecules. KE = ½ mv2 Does 0°C really mean 0 KE? nope... it simply means the freezing point of water, a convenient standard. We have to cool things down to –273.15°C before we reach 0 KE. This is called 0 Kelvin (0 K, note: NO ° symbol.) For phenomena that are proportional to the KE of the particles (pressure of a gas, etc.) you must use temperatures in K. K = °C + 273 °C = K – 273 °F = 9/5 °C + 32 °C = 5/9 (°F – 32) Note: In Kelvin notation, the degree sign is omitted: 283K Celsius to Fahrenheit: *A mental shortcut for a rough estimate: · Double the temperature given in Celsius · Add 30 to the result to find the approximate temperature in Fahrenheit. Celsius Temperature to Fahrenheit More Celsius to Fahrenheit Fahrenheit to Celsius More Fahrenheit to Celsius Fahrenheit to Celsius: *A mental shortcut for a rough estimate: ·Subtract 30 from the temperature given in Fahrenheit · Take half of the result to find the approximate temperature in Celsius. Celsius Temperature to Fahrenheit More Celsius to Fahrenheit Fahrenheit to Celsius More Fahrenheit to Celsius Energy is measured in many ways. BTU One of the basic measuring blocks is called a Btu. This stands for British thermal unit and was invented by the English. Btu is the amount of heat energy it takes to raise the temperature of one pound of water by one degree Fahrenheit, at sea level. ◦ One Btu equals about one blue-tip kitchen match. ◦ One thousand Btus roughly equals: One average candy bar or 4/5 of a peanut butter and jelly sandwich. ◦ It takes about 2,000 Btus to make a pot of coffee. A calorie is a unit of measurement for energy. Calorie is a French word derived from the Latin word: calor (heat). Modern definitions for calorie fall into two classes: The small calorie or gram calorie approximates the energy needed to increase the temperature of 1 gram of water by 1 °C. This is about 4.184 joules. The large calorie or kilogram calorie approximates the energy needed to increase the temperature of 1 kg of water by 1 °C. This is about 4.184 kJ, and exactly 1000 small calories. 1 cal = 4.184 J Energy also can be measured in joules. (Joules sounds exactly like the word jewels, as in diamonds and emeralds.) A thousand joules is equal to a British thermal unit. 1,000 joules = 1 Btu So, it would take 2 million joules to make a pot of coffee. The term "joule" is named after an English scientist James Prescott Joule who lived from 1818 to 1889. He discovered that heat is a type of energy. One joule is the amount of energy needed to lift something weighing one pound to a height of nine inches. Around the world, scientists measure energy in j. Like in the metric system, you can have kilojoules -- "kilo" means 1,000. 1,000 joules = 1 kilojoule = 1 Btu 1 cal = 4.184 J Temperature is a measure of the Average kinetic energy of the molecules of a substance. Higher temperature faster molecules. At absolute zero (0 K) all molecular motion would stop. High temp. % o f M o l e c u l e s Low temp. Kinetic Energy % o f M o l e c u l e s High temp. Low temp. Average kinetic energies are temperatures Kinetic Energy The average kinetic energy is directly proportional to the temperature in Kelvin If you double the temperature (in Kelvin) you double the average kinetic energy. If you change the temperature from 300 K to 600 K the kinetic energy doubles. If you change the temperature from 300ºC to 600ºC the Kinetic energy doesn’t double. 873 K is not twice 573 K Melting Solid Vaporization Liquid Freezing Gas Condensation endothermic Sublimation Melting Vaporization Solid Liquid Freezing Gas Condensation Deposition exothermic Heating Curve for Water 120 Steam Water and Steam 100 80 60 Water 40 20 0 Ice Water and Ice -20 0 40 120 220 760 800 Heating Curve for Water 120 Water and Steam 100 gas Steam 80 liquid 60 Water 40 20 0 Ice Solid -20 0 40 Slope = Specific Heat Water and Ice 120 220 760 800 Heating Curve for Water 120 Steam Water and Steam 100 80 60 Water 40 20 Both Solid Water and Ice Iceand liquid 0 -20 0 40 120 220 760 800 Heating Curve for Water 120 BothWater liquid and and Steam gas 100 80 Steam 60 Water 40 20 0 Ice Water and Ice -20 0 40 120 220 760 800 Heating Curve for Water 120 Heat ofand Water Steam Vaporization 100 Steam 80 60 Water 40 20 0 Ice Water and Ice -20 0 40 120 220 760 800 Heating Curve for Water 120 Steam Water and Steam 100 80 60 Water 40 20 Heat ofWater Ice Fusionand Ice 0 -20 0 40 120 220 760 800 Heating Curve for Water 120 Steam Water and Steam 100 80 60 Water 40 Plateau = phase equilibrium 20 0 Ice Water and Ice -20 0 40 120 220 760 800 43 The specific heat capacity (C) determines the rate at which heat will be absorbed. Even though mass is present in the formula it is an intensive property like density and is unique for each substance. The specific heat capacity for water is 4.18J/g The quantity of heat absorbed (Q) can be calculated by: Q=mCT m=mass T=change in temperature J Deutsch 2003 44 Heat capacity is an extensive property, meaning it depends on the mass of the object. Ex: 1000g of water can hold more heat than 10 g of water. Q means heat energy lost or gained. Law of Conservation of Mass-Energy m= mass of substance; Cp= specific heat capacity; T = change in temperature Qlost = Qgained Three equations: Q= mass x Cp x T Q= Hf x mass Q= Hv x mass Heating Curve for Water 120 Steam Water and Steam 100 80 60 Water 40 20 0 Ice Water and Ice -20 0 40 120 220 760 800 Heat of fusion energy required to change one gram of a substance from solid to liquid. (endothermic rxn) Heat of solidification energy released when one gram of a substance changes from liquid to solid. (exothermic rxn) For water 80 cal/g or 334 J/g Heat of vaporization energy required to change one gram of a substance from liquid to gas. (endothermic rxn) Heat of condensation energy released when one gram of a substance changes from gas to liquid. (exothermic rxn) For water 540 cal/g or 2260 J/g Three equations: Q= mass x Cp x T (used at slopes) Q= Hf x mass Q= Hv x mass (used at s/l equilibria) (used at l/g equilibria) Heating Curve for Water 120 Steam Water and Steam 100 80 60 Water 40 20 0 Ice Water and Ice -20 0 40 120 220 760 800 As ice melts at standard pressure, its temperature remains at 0°C until it has completely melted. Its potential energy (1) decreases (2) increases (3) remains the same 52 A sample of water is heated from a liquid at 40°C to a gas at 110°C. The graph of the heating curve is shown in your answer booklet. a On the heating curve diagram provided in your answer booklet, label each of the following regions: Liquid, only Gas, only Phase change Gas Only Phase change Liquid Only 53 b For section QR of the graph, state what is happening to the water molecules as heat is added. They move faster, their temperature increases. c For section RS of the graph, state what is happening to the water molecules as heat is added. Their intermolecular bonds are breaking, their potential energy is increasing. 54 What is the melting point of this substance? (1) 30°C (3) 90°C (2) 55°C (4) 120°C 55 Melting (fusion) or freezing (solidification) ◦ Q=mHf where Hf is the heat of fusion (for water: 333.6 J/g) Boiling (vaporization) or condensing ◦ Q=mHv where Hv is the heat of vaporization (for water: 2259 J/g) Hf and Hv are given to Table B – m is the mass 56 In which equation does the term “heat” represent heat of fusion? (1) NaCl(s) + heat NaCl(l) (2) NaOH(aq) + HCl(aq) NaCl(aq) + H2O(l)+ heat (3) H2O(l)+ heat H2O(g) (4) H2O(l)+ HCl(g) H3O+(aq) + Cl –(aq) + heat Fusion refers to melting. J Deutsch 2003 57 The temperature at which a liquid and a solid are in equilibrium The melting point for ice is 0ºC The melting point of a substance is the same as its freezing point J Deutsch 2003 58 The solid and liquid phases of water can exist in a state of equilibrium at 1 atmosphere of pressure and a temperature of (1) 0°C (2) 100°C (3) 273°C (4) 373°C J Deutsch 2003 59 The total heat = the sum of all the heats you have to use Go in order Heat Ice Below 0 C + Melt Ice At 0 C + Heat Water + 0 C - 100 C Boil Water + At 100 C Heat Steam Above 100 C Heating Curve for Water 120 Steam Water and Q Steam + 100 80 Q + 60 Q 40 Water + 20 0 Q+ Ice -20 0 40 Q Water and Ice 120 220 760 800 Heating Curve for Water Q=m x Cp x ∆T Steam Water and Q= Hv Steam xm 120 100 80 Q=m x Cp x ∆T 60 Water 40 20 Q= Water Hf x m and Ice Ice Q=m x Cp x ∆T 0 -20 0 40 120 220 760 800