to the Lecture PowerPoint for this chapter
advertisement

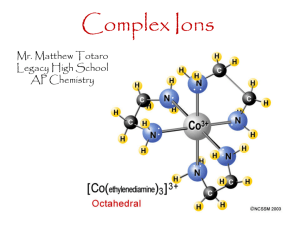
Chemistry: A Molecular Approach, 1st Ed. Nivaldo Tro Chapter 24 Transition Metals and Coordination Compounds Roy Kennedy Massachusetts Bay Community College Wellesley Hills, MA 2007, Prentice Hall Gemstones • the colors of rubies and emeralds are both due to the presence of Cr3+ ions – the difference lies in the crystal hosting the ion Some Al3+ ions in Al2O3 are replaced by Cr3+ Tro, Chemistry: A Molecular Approach Some Al3+ ions in Be3Al2(SiO3)6 are replaced by Cr3+ 2 Properties and Electron Configuration of Transition Metals • the properties of the transition metals are similar to each other and very different tot he properties of the main group metals high melting points, high densities, moderate to very hard, and very good electrical conductors • in general, the transition metals have two valence electrons – we are filling the d orbitals in the shell below the valence Group 1B and some others have 1 valence electron due to “promotion” of an electron into the d sublevel to fill it form ions by losing the ns electrons first, then the (n – 1)d Tro, Chemistry: A Molecular Approach 3 Atomic Size • the atomic radii of all the transition metals are very similar small increase in size down a column Tro, Chemistry: A Molecular Approach 4 Ionization Energy • the first ionization energy of the transition metals slowly increases across a series • third transition series slightly higher 1st IE trend opposite to main group elements Tro, Chemistry: A Molecular Approach 5 Electronegativity • the electronegativity of the transition metals slowly increases across a series except for last element in the series • electronegativity slightly increases down the column trend opposite to main group elements Tro, Chemistry: A Molecular Approach 6 Oxidation States • often exhibit multiple oxidation states • vary by 1 • highest oxidation state is group number for 3B to 7B Tro, Chemistry: A Molecular Approach 7 Coordination Compounds • when a complex ion combines with counterions to • • make a neutral compound it is called a coordination compound the primary valence is the oxidation number of the metal the secondary valence is the number of ligands bonded to the metal coordination number • coordination number range from 2 to 12, with the most common being 6 and 4 CoCl36H2O = [Co(H2O)6]Cl3 Tro, Chemistry: A Molecular Approach 8 Coordination Compound Tro, Chemistry: A Molecular Approach 9 Complex Ion Formation • complex ion formation is a type of Lewis acidbase reaction • a bond that forms when the pair of electrons is donated by one atom is called a coordinate covalent bond Tro, Chemistry: A Molecular Approach 10 Ligands with Extra Teeth • some ligands can form more than one coordinate covalent bond with the metal atom lone pairs on different atoms that are separate enough so that both can reach the metal • chelate is a complex ion containing a multidentate ligand ligand is called the chelating agent Tro, Chemistry: A Molecular Approach 11 Tro, Chemistry: A Molecular Approach 12 EDTA a Polydentate Ligand Tro, Chemistry: A Molecular Approach 13 Complex Ions with Polydentate Ligands Tro, Chemistry: A Molecular Approach 14 Geometries in Complex Ions Tro, Chemistry: A Molecular Approach 15 Naming Coordination Compounds 1) determine the name of the noncomplex ion 2) determine the ligand names and list them in 3) 4) alphabetical order determine the name of the metal cation name the complex ion by: 1) name each ligand alphabetically, adding a prefix in front of each ligand to indicate the number found in the complex ion 2) follow with the name of the metal cation 5) write the name of the cation followed by the name of the anion Tro, Chemistry: A Molecular Approach 16 Common Ligands Tro, Chemistry: A Molecular Approach 17 Common Metals found in Anionic Complex Ions Tro, Chemistry: A Molecular Approach 18 Isomers • Structural isomers are molecules that have the same number and type of atoms, but they are attached in a different order • Stereoisomers are molecules that have the same number and type of atoms, and that are attached in the same order, but the atoms or groups of atoms point in a different spatial direction Tro, Chemistry: A Molecular Approach 19 20 Linkage Isomers Tro, Chemistry: A Molecular Approach 21 Geometric Isomers • geometric isomers are stereoisomers that differ in the spatial orientation of ligands • cis-trans fac-mer isomerism isomerismin inoctahedral octahedralcomplexes square-planar complexes complexes MA MAMA B322B2 34B Tro, Chemistry: A Molecular Approach 22 Ex. 24.5 – Draw the structures and label the type for all isomers of [Co(en)2Cl2]+ the ethylenediamine ligand (en = H2NCH2CH2NH2) is bidentate each Cl ligand is monodentate octahedral MA4B2 Tro, Chemistry: A Molecular Approach 23 [Co(en)3]3+ Optical Isomers • optical isomers are stereoisomers that are nonsuperimposable mirror images of each other Tro, Chemistry: A Molecular Approach 24 Ex 24.7 – Determine if the cis-trans isomers of [Co(en)2Cl2]+ are optically active • draw the mirror image of the given isomer and check to see if they are superimposable cis isomer trans isomer mirroridentical image istononsuperimposable its mirror image nooptical opticalisomers isomerism Tro, Chemistry: A Molecular Approach 25 Bonding in Coordination Compounds Valence Bond Theory • bonding take place when the filled atomic orbital on the ligand overlaps an empty atomic orbital on the metal ion • explain geometries well, but doesn’t explain color or magnetic properties Tro, Chemistry: A Molecular Approach 26 Tro, Chemistry: A Molecular Approach 27 Bonding in Coordination Compounds Crystal Field Theory • bonds form due to the attraction of the electrons on the • • • ligand for the charge on the metal cation electrons on the ligands repel electrons in the unhybridized d orbitals of the metal ion the result is the energies of orbitals the d sublevel are split the difference in energy depends the complex and kinds of ligands crystal field splitting energy strong field splitting and weak field splitting Tro, Chemistry: A Molecular Approach 28 Splitting of d Orbital Energies due to Ligands in a Octahedral Complex Tro, Chemistry: A Molecular Approach 29 Strong and Weak Field Splitting Tro, Chemistry: A Molecular Approach 30 Complex Ion Color • the observed color is the complimentary color of the one that is absorbed Tro, Chemistry: A Molecular Approach 31 Complex Ion Color and Crystal Field Strength • the colors of complex ions are due to electronic transitions between the split d sublevel orbitals • the wavelength of maximum absorbance can be used to determine the size of the energy gap between the split d sublevel orbitals Ephoton = hn = hc/l = D Tro, Chemistry: A Molecular Approach 32 Ligand and Crystal Field Strength • the strength of the crystal field depends in large part on the ligands strong field ligands include: CN─ > NO2─ > en > NH3 weak field ligands include H2O > OH─ > F─ > Cl─ > Br─ > I─ • crystal field strength increases as the charge on the metal cation increases Tro, Chemistry: A Molecular Approach 33 Magnetic Properties and Crystal Field Strength • the electron configuration of the metal ion with split d • • orbitals depends on the strength of the crystal field the 4th and 5th electrons will go into the higher energy dx2-y2 and dz2 if the field is weak and the energy gap is small – leading to unpaired electrons and a paramagnetic complex the 4th thru 6th electrons will pair the electrons in the dxy, dyz and dxz if the field is strong and the energy gap is large – leading to paired electrons and a diamagnetic complex Tro, Chemistry: A Molecular Approach 34 Low Spin & High Spin Complexes diamagnetic paramagnetic low-spin complex high-spin complex only electron configurations d4, d5, d6, or d7 can have low or high spin Tro, Chemistry: A Molecular Approach 35 Tetrahedral Geometry and Crystal Field Splitting • because the ligand approach interacts more strongly with the planar orbitals in the tetrahedral geometry, their energies are raised • most high-spin complexes Tro, Chemistry: A Molecular Approach 36 Square Planar Geometry and Crystal Field Splitting • d8 metals • the most complex splitting pattern • most are low-spin complexes Tro, Chemistry: A Molecular Approach 37 Applications of Coordination Compounds • extraction of metals from ores silver and gold as cyanide complexes nickel as Ni(CO)4(g) • use of chelating agents in heavy metal poisoning EDTA for Pb poisoning • chemical analysis qualitative analysis for metal ions blue = CoSCN+ red = FeSCN2+ Tro, Chemistry: A Molecular Approach 38 Applications of Coordination Compounds • commercial coloring agents prussian blue = mixture of hexacyanoFe(II) and Fe(III) inks, blueprinting, cosmetics, paints • biomolecules porphyrin ring cytochrome C hemoglobin chlorphyll Tro, Chemistry: A Molecular Approach chlorophyll 39 Applications of Coordination Compounds • carbonic anhydrase catalyzes the reaction between water and CO2 contains tetrahedrally complexed Zn2+ Tro, Chemistry: A Molecular Approach 40 Applications of Coordination Compounds • Drugs and Therapeutic Agents cisplatin anticancer drug Tro, Chemistry: A Molecular Approach 41