Final Exam Review * SNC 2D1
advertisement

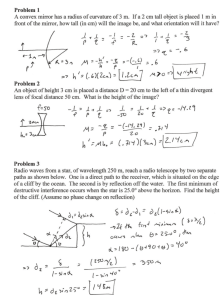
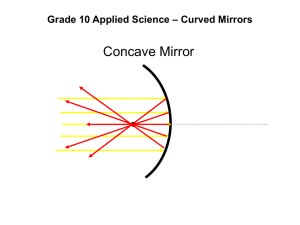
SNC 2D Final Exam Review – SNC 2D DATE: Wed January 28, 2015 TIME: 8:00 am- Room 206 DURATION: 1.5 hrs, 112 marks Category Suggested Time Multiple Choice (Knowledge) Matching (Knowledge) Diagrams (Application) Short Answer (Application) Total Marks 20 min 35 15 min 16 15 min 20 30 min 41 1hr 20 min (10 min to look over) 112 Helpful Hints * Organize your notes * Make sure you have ALL the handouts and notes * Make sure all of your quizzes, tests, and assignments are corrected (solutions available online) * Make summary notes (compress your notes so you only have a handful of papers to study from) * Make sure you have all diagrams Prepare * Analyze your past test/quiz/lab results Where did you go wrong? How can you improve? * Stay relaxed and confident Don’t talk about the exam to others before you write. Anxiety is contagious. Reviewing * Take good notes (make sure everything is correct) * Organize your notes * Estimate the hours it will take to review (time management) * Create a study schedule Study in chunks, don’t try and study everything everyday * Test yourself or a friend Study by yourself, then get a friend to quiz you Helping other classmates also helps you get a better understanding of the material * Finish your studying the day BEFORE the exam - Cramming is not good Get at least 8 hrs of sleep the night before. Have a healthy breakfast and relax Test Taking * Read directions carefully * Do a quick overview of the exam before you start writing * Answer questions in a strategic order Easy first - builds confidence and may help you make associations with the harder questions Put a * beside questions you are unfamiliar with and come back to them later * Make sure you look at marks allocation and have enough points to get full marks * A question worth 3 marks will probably require 3 relevant points * Review Make sure you have completed all the questions to the best of your ability Make sure you have answered all the questions. Never leave a question blank Make a common sense guess, which could give you more marks than if left blank SNC 2D Write something that you can remember about the topic Look for any mistakes you have made Multiple Choice * Do the questions you know first * Read the question a couple of times to make sure you understand it * Look for key words (always, never, not, etc) * Process of elimination (cross off answers you know are wrong) Biology Exam review 1. Plant and animal cells – differences and similarities – know the parts of the cell (organelles) and the function of each part 2. Know cellular respiration 3. Know the cell theory and cell organization pyramid 4. Know the different types of cells/tissues: muscle, nervous, fat, stem etc. 5. Know the locations and functions of the various tissues --- structure and function are always related 6. Mitosis – know the cell cycle – interphase, prophase, metaphase, anaphase, telophase and cytokinesis 7. Be familiar with what is happening to the chromosomes/sister chromosomes, centrioles, spindle fibres, centromeres, nuclear membrane, cytoplasm, cell wall in each of the stages of mitosis (prophase, metaphase, anaphase and telophase) 8. Know the heart, the path of oxygenated blood/deoxygenated blood in the heart. Know which vessels lead to/return from the lungs and which lead to/return from body 9. Human systems – skeletal, digestive, circulatory and respiratory, nervous-how they interact with each other a. Know the parts/organs, the functions of each part, the pathway (e.g – that food takes through the digestive system), the functions/jobs/purposes of each system 10. Know the parts of the plants: specifically the different types of tissue and their functions Optics Exam Review 11. Wave Theory of Light and the Electromagnetic Spectrum/properties of light. Wavelengths of light 12. Be familiar with different sources of light (e.g. bioluminescence, chemiluminescence, phosphorescence) 13. Types of objects – transparent, translucent, opaque, etc. 14. Reflection – point of incidence, normal, incident ray, angle of incidence, reflected ray, angle of reflection, the “plane” where all these lie. Know the law of reflection: <I =<R 15. Reflection in a… a. Plane mirror – know the two methods to locate an image in the mirror i. SALT – characteristics to describe the image b. Concave/convex mirrors – 3 incidence rays are used to locate the image i. SALT – characteristics to describe the image c. Concave/Convex lenses – 3 incidence rays are used to locate the image i. SALT – characteristics to describe the image 16. Ray diagrams – plane, concave, convex mirror, convex and concave lenses 17. Know which lens/mirror converges light and which diverges light 18. Calculations – magnification (using height and distance), refraction problems, thin lens equation 19. Know about the refraction of light. Does the light bend toward or away from the normal as it passes from a less dense medium (e.g. air to water or air to glass)? What happens when it goes from a more to a less dense medium 20. Know the index of refraction formula and how to use it in a word problem. Make sure you list your givens and your unknowns, then write down the formula and fill in your givens. Solve for the unknown. SNC 2D Chemistry Exam Review 21. What are 5 indicators that a chemical reaction has occurred? 22. Be familiar with the particle theory 23. Know the general structure of the atom – proton, neutron, electron 24. Know the specific structures of the first 20 elements – you will be given a periodic table from which you can get atomic number and atomic mass (know what an isotope is) – know how to determine the number of protons, electrons and neutrons given the atomic number and atomic mass 25. Be able to draw Bohr-Rutherford and Lewis dot/electron diagrams for any given atom 26. What is an ion? Positive ion? Negative ion? 27. Know the differences between ionic and covalent/molecular compounds. Know the different properties they possess 28. Nomenclature (chemical formula to name, name to chemical formula) a. Ionic Compounds i. Multivalence ions ii. Polyatomic ions b. Molecular Compounds 29. Physical and chemical changes – recognizing chemical change 30. State the law of conservation of mass and explain how this relates to balancing chemical equations. 31. Balancing equations (word and skeletal) 32. Types of chemical reactions (sd, dd, decomposition, synthesis, combustion) a. Know how to recognize the types of reactions and how to determine the products 33. Acids and bases a. pH scale b. How do you know if it is an acid or a base c. Universal pH paper, red litmus, blue litmus 34. Neutralization reactions: what are they? What would the reactants be? The products? Climate Exam Review 35. The difference between weather and climate. Examples of each 36. What is the atmosphere. What is it made up of 37. How does the radiation from the sun affect particles in the atmosphere? On earth? 38. The balance of the suns radiation and what is absorbed on the earth and what is reflected. Why this balance is important 39. What items absorb, reflect or radiate the suns radiation 40. Greenhouse gasses, what are they? 41. Greenhouse effect and Anthropogenic greenhouse effect 42. Human activities that contribute to the greenhouse effect 43. What is the Ozone? 44. The hydrosphere and the hydro-cycle 45. Feedback loops Balance each of the following & identify the type of reaction 1. 2. 3. 4. 5. 6. 7. 8. 9. NaCl(aq) N02(g) NH4 0H(aq) Fe203(S) Sb(s) HBr(aq) N02(g) Na2C03(S) CaC03(S) + + + + + + + + H20(l) 02(g) C02(g) C0(g) Cl2(g) Cl2(g) H20(l) Ca(0H)2(S) Ca0(S) + Na0H(aq) + N03(g) (NH4)2C03(aq) + Fe(S) + SbCl3(S) HCl(aq) + HNO3(aq) + Na0H(S) + C02(g) Cl2(g) H20(l) C02(g) Br2(l) N0(g) CaC03(S) SNC 2D NAMING IONIC & COVALENT COMPOUNDS Name of Compound Formula Name of Compound Formula Cr2(SO4)3 Iron (III) chloride carbon tetrachloride Ba3(PO4)2 Calcium sulfate P4O6 Potassium sulfide N2O4 boron trifluoride PbS2 Fe2Se3 Magnesium hydroxide Magnesium nitride Hg2Cr2O7 lead(IV) oxide MnF4 manganese(IV) oxide Ag2SO4 stannic nitrate SO2 chromium(III) oxide N2O3 dinitrogen oxide PuO2 sodium dichromate Au2(CrO4)3 aluminum hydroxide AlI3 calcium bisulfate CrSO3 SNC 2D Mirror & Lens Diagram Worksheet 2 Complete each diagram to determine the image that will result. Make sure that you describe each image using “SALT”. S: A: L: T: Object beyond C Object at C Object between C and F Object at F SNC 2D Object between F and mirror Convex Mirror