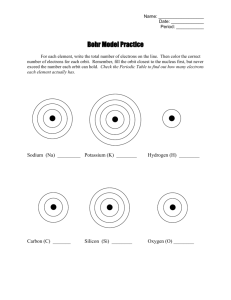
Bohr Model of the Atom
advertisement

Electrons in Atoms Chapter 5 What were the early steps in the development of atomic theory? • John Dalton – Billiard Ball Theory. Atom was indivisible. • J.J. Thomson – Plum Pudding Model. Atom was composed of smaller particles, including negative electrons. • E. Rutherford – Nuclear Model. Rutherford Model • Nucleus contains all positive charge & most of the mass. • Nucleus is very small in volume - only 1/10,000th of atomic diameter. • Electrons occupy most of the volume of the atom. Later Models • N. Bohr – Planetary Model • Schrodinger – Wave Mechanics + - - - - +6 - - source Cathode Ray Tubes & Discovery of Electrons source Movie of cathode ray tube source Rutherford’s Experiment - 1911 source Discharge Tubes (Neon Lights) Problems with the Rutherford Model • Why doesn’t the electron spiral into the nucleus? • How are the electrons arranged? • Why do different elements exhibit different chemical behavior? • How are the atomic emission spectra produced? Atomic Emission Spectra • Put some gas in a glass tube and apply a voltage across the ends. Produce light. • The color of the light depends on the gas in the tube. • Every element produces its own unique color. The emission spectrum of an element is the set of frequencies (or wavelengths) emitted. Atomic Emission Spectra: Line spectra Why is the emission spectra useful? • We can use it to determine if a given element is present in a sample. (Identification!) • We can use it to learn fundamental information about atomic structure. • Neon lights Quick light review • Energy of light = E = h • In the visible spectrum, – Blue light: shorter wavelength, higher frequency, more energy – Red light: longer wavelength, lower frequency, less energy Interlude: Electromagnetic Radiation All electromagnetic radiation has a velocity of 3.00 X 108 m/sec c = Emission & Absorption Spectra of Elements Each line in the bright line spectrum represents 1 electron jump. The blue lines have shorter wavelength and higher frequency, so they are light from “bigger” jumps. The red lines have longer wavelength and lower frequency, so they are light from “smaller” jumps. Bohr Diagram • Shows all the electrons in orbits or shells about the nucleus. n=3 n=2 n=1 E3 E2 E1 Bohr’s Model •Electrons travel only in specific orbits. •Each orbit has a definite energy. source • The energy of the electron must match the energy of the orbit. So the electron is only allowed to have some energies, not any energy. NEW! Bohr assigned a quantum number, n, to each orbit. Bohr’s Model •The orbit closest to nucleus is the smallest & has the lowest energy. It has n = 1. •Outer orbits hold more electrons than inner orbits. source •The larger the orbit, the more energy associated with it. Bohr’s Model •Atoms emit radiation when an electron jumps from an outer orbit to an inner orbit. •Atoms absorb energy when an electron jumps from an inner orbit to an outer orbit. source Bohr Applet •Outer orbits determine atom’s chemical properties. Bohr Diagram n=3 n=2 n=1 * Made-up Numbers!!! E3 = 23* E2 = 15* E1 = 5* What is the energy change of the electron if it moves from E1 to E3? It must absorb 18 units of energy. From E2 to E1? It must release 10 units of energy. Bohr Model • Energy is absorbed when the electron moves to a higher orbit, farther from nucleus. Endothermic process. • Energy is released when the electron drops to a lower orbit, closer to nucleus. Exothermic process. Hydrogen Atom Flame Tests • Volume 2, CCA – – – – Na Sr Cu Animation The energy levels get closer together away from the nucleus. Larger orbits can hold more electrons. Emitted Light • The energy of the emitted light, E = h, matches the difference in energy between 2 levels. • Again, we don’t know the absolute energy of the energy levels, but we can observe how far apart they are from each other. source source Tiger Graphic – potential energy Tiger Graphic – electron orbits Potential Energy A ladder is often used as an analogy for the energy levels of an atom. But it’s a little bit different – How? Max Capacity of Bohr Orbits Orbit Max # of Electrons 1 2 2 8 3 18 4 32 n 2n2 Ground State • Each electron is in the lowest energy orbit available. Lowest energy state of an atom. • The Bohr configurations in the reference table are ground state configurations. Excited State • Many possible excited states for each atom. One or more electrons excited to a higher energy level. • We can give electron configurations for the excited states as well as the ground state. • You need to recognize excited state configurations. Excited State Configurations • For the smaller elements, it’s easy. – First level holds 2. – Second level holds 8. – If upper levels fill before the first or second is full, it’s an excited state configuration. Some Excited States for Li • Ground State of Li is 2-1. • • • • • 2-0-1 2-0-0-1 1-2 1-1-1 1-1-0-1 Each possible configuration still has 3 electrons, but now one electron has been bumped up to a higher level. Many other possibilities! Excited State Configurations • Which is an excited state configuration of Fluorine, F? • Fluorine is element 9. a) 2-7 Matches g.s. b) 2-8 10 c) 2-6 d) 1-8 8 Excited State Configurations • Which is an excited state configuration of S? • S is element 16. a) 2-7-6 15 b) 2-8-6 G.S. c) 2-7-8 17 d) 2-7-7 Excited State Configurations Determine which of the following is an excited state configuration of Manganese, Mn. Manganese is element 25. a) 2-8-13-1 24 c) 2-7-14-2 b) 2-8-13-3 26 d) 2-8-13-2 Matches g.s. Excited State Configurations • For the larger elements, the best thing to do is compare the given configuration with the ground state configuration in the reference tables. – Note: The configuration must have the correct number of electrons for that element. – If the configuration matches the one in the reference table, it’s ground state. – If it does NOT match, it’s excited state. Electron Transitions • If an electron gains or absorbs a specific amount of energy, it can be excited to a higher energy level. • If an electron loses or emits a specific amount of energy, it moves down to a lower energy level. Emitted Light • The energy of the emitted light, E = h, matches the difference in energy between 2 levels. • Turns out, we don’t know the absolute energy of the energy levels, but we can observe how far apart they are from each other. Hydrogen has 1 electron, but it can make many possible transitions. Potential Energy A ladder is often used as an analogy for the energy levels of an atom. But it’s a little bit different – How? source source Tiger Graphic – potential energy Tiger Graphic – electron orbits Absorption & Emission • We cannot easily detect the absorption of energy by the electron. • We can easily detect the emission of energy by the electron. We can see the photon that is kicked out. Success of Bohr’s Model • Bohr’s model could predict the frequencies in the hydrogen emission spectrum. • Predicted correct size of H atom. • Unfortunately, it didn’t work for anything with more than 1 electron. Which principal energy level of an atom contains an electron with the lowest energy? a) b) c) d) n=1 n=2 n=3 n=4 What is the total number of occupied principal energy levels in an atom of neon in the ground state? a) b) c) d) 1 2 3 4 What is the total number of fully occupied principal energy levels in an atom of nitrogen in the ground state? a) b) c) d) 1 2 3 4 What is the total number of electrons in a completely filled fourth principal energy level? a) b) c) d) 8 10 18 32 Which atom in the ground state has five electrons in its outer level and 10 electrons in its kernel? a) b) c) d) C Cl Si P Kernel? • Everything EXCEPT the valence electrons. • = the nucleus + ALL the INNER SHELL electrons. Which electron configuration represents an atom in an excited state? a) b) c) d) 2-8-2 2-8-1 2-8 2-7-1 Which electron configuration represents an atom of Li in an excited state? a) b) c) d) 1-1 1-2 2-1 2-2 The characteristic bright-line spectrum of an atom is produced by its a) b) c) d) Electrons absorbing energy Electrons emitting energy Protons absorbing energy Protons emitting energy