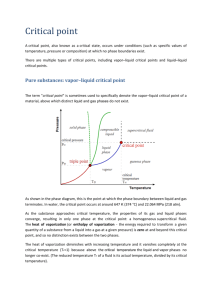
Pure Substance 2015 10 25
advertisement

PURE SUBSTANCE I am teaching Engineering Thermodynamics using the textbook by Cengel and Boles. Many figures in the slides are taken from that book, and most others are found online. Similar figures can be found in many places. I went through these slides in two lectures, each 90 minutes. Zhigang Suo Pure Substance • • A substance: a collection of molecules or atoms A pure substance: A substance that has a homogeneous composition. • • To say something is homogeneous requires us to specify a length scale and a time scale. A tank of air is homogeneous over a length larger than that between molecules, and over a time larger than that between collisions. Below such a length scale and a time scale, the substance is inhomogeneous. • 2 Liquid-gas mixture 3 Phase One species of molecules can aggregate into several forms, known as phases. ice water steam Solid liquid gas 4 System • A system can be any part of the world. • The rest of the world is called the surroundings of the system. weights Experimental setup: cylinder-piston device • A fixed number of H2O molecules • Cylinder, rigid. • Piston, frictionless. • Weights • Fire vapor liquid fire 5 Isolated system • An isolated system does not interact with the rest of the world. • Seal the cylinder-piston device, so that the number of H2O molecules in the device is fixed. • Jam the piston, so that the volume in the device is fixed. • Insulate the cylinder and the piston, so that the device and the surroundings do not exchange energy by heat. • Do whatever necessary to prevent the rest of the world from affecting the system. weights vapor vapor liquid liquid fire isolated system 6 State • A system isolated for a long time reaches a state of thermodynamic equilibrium. • In a state of thermodynamic equilibrium, the system appears to be static at a macroscopic scale, but molecules keep moving. • Synonyms: state, state of equilibrium, thermodynamic state, state of thermodynamic equilibrium. • The system (a fixed number of H2O molecules) can be in many states. • Fire and weights transform (change) the system from one state to another. • The transformation between two states is known as a thermodynamic process. weights vapor vapor liquid liquid fire isolated system 7 Property • • • • A function of state is called a thermodynamic property (variable). Examples: temperature, pressure, volume, energy, entropy… Name all thermodynamic states of a pure substance using two properties, TV Once the values of the two independent variables are fixed, a state of the system is fixed. • Any other property is a function of the two independent variables. P(T,V) weights vapor vapor liquid liquid fire isolated system 8 Intensive and extensive properties • • • • For a fluid in a state of thermodynamic equilibrium, the temperature is everywhere the same, and the pressure is everywhere the same. Temperature and pressure are intensive properties. The volume of a system equals the sum of the volumes of all parts of the system. Volume is an extensive property. 9 Specific volume volume of system) ( (specific volume) = mass of system ( ) V v= m ( 1 specific volume = density ) ( ) 10 High-school mathematics Four ways to represent a function of two independent variables, z(x,y) • • • • Contour plot (plane diagram) Table A surface in 3D An equation 11 compressed liquid saturated liquid coexistent Liquid and vapor saturated vapor superheated vapor a States • Specify states with two variables, T and v • Change of state • Continuous change of state Phases • Two phases: liquid and gas • Change of phase • Discontinuous change of state • A state of coexistent phases: liquid-gas mixture 12 Represent states on the T-v plane • • • • Specify states with two variables, T and v. Represent a state by a point on the T-v plane. Pressure is a function, P (T,v) Represent the function P (T,v) on the T-v plane by curves of constant pressure (isobaric curves) weights vapor liquid fire 13 The discovery of the dome A point inside the dome specifies a state of coexistent phases. Thomas Andrews, On the continuity of the gaseous and liquid states of matter. Philosophical Transactions of the Royal Society of London 159, 575-590 (1869) 14 Two paths to change from one state to another state A path of continuous change of state A path of discontinuous change of state 15 A state of coexistent phases Specify a state of coexistent phases by values of two variables (T,v) or (P,v), but not (P,T). Define quality by x = mgas mgas + mliq T 0 < x < 1: a mixture of liquid and vapor x = 0: saturated liquid x = 1: saturated vapor v vf Volume and specific volume ( v vg vf specific volume of saturated liquid vg specific volume of saturated gas v specific volume of a mixture of liquid and gas ) V = mgas + mliq v, Vliq = mliqv f , Vgas = mgasvg Volume is additive V = Vliq +Vgas ( ) Specific volume follows rule of mixture v = 1 - x v + xv f g Two more ways to specify a state of coexistent phases: (T,x) or (P,x). 16 Heat causes giant motion when liquid changes to gas P = 100 kPa Tsat = 100 degC Vf = 10-3 m3/kg Vg = 1.7 m3/kg https://www.ohio.edu/mechanical/thermo/Intro/Chapt.1_6/Chapter2a.html 17 Represent states on PV 18 https://www.ohio.edu/mechanical/thermo/Intro/Chapt.1_6/Chapter2a.html 19 Saturation Temperature and Saturation Pressure liquid gas 20 Two paths to change from one state to another state A path of discontinuous change of state A path of continuous change of state a a P P critical point critical point liquid a a liquid gas gas T a T a 21 22 Pressure cooker Invented by Denis Papin, France, 1679 Invention: increase pressure, increase temperature, reduce cooking time. Science: When water and steam coexist, temperature increases with time. Engineering: seal, strength, control pressure or temperature. P ~ 2 atm T ~ 120 dedC 23 Bottled gas by liquefaction Invention: store gas in small volume, at room temperature . Science: At room temperature and high pressure, some gases become liquids. Engineering: seal, strength. No need for thermal insulation. Ammonia, NH3 liquid gas 24 Fix temperature by using boiling point Invention: Fix temperature by using boiling points of various liquids. Science: When a liquid evaporates at the atmospheric pressure, the temperature is fixed. Engineering: seal, insulation. 25 Tables of properties inside the dome coexistent liquid and vapor A partial list of Table A–4. • Table A–4: Saturation properties of water under temperature. • Table A–5: Saturation properties of water under pressure. 26 Tables of properties outside the dome Compressed liquid or superheated vapor Specify a state by values of PT A partial listing of Table A–6. 27 A bit of high-school science Ideal-gas law Pv = RT Equation of state: An equation that relates properties of a substance. 28 Is Water Vapor an Ideal Gas? 29 Principle of corresponding states • • • • Use PT as independent variables. Normalize them by critical vales. Any property is a function of the two independent variables. Pv/RT is a (dimensionless) property. æ P T ö Pv ÷÷ = f çç , RT è Pcr Tcr ø TR = T /Tcr Pv RT • • • • P / Pcr At low pressure, and all temperatures, all substances approach to ideal gas, Pv/RT ~ 1. At high temperature, and all pressures, all substances approach to ideal gas, Pv/RT ~ 1. Any property is a function of the two independent variables. The function Pv/RT = f(P/Pcr, T/Tcr) is nearly the same for all substances. 30 van der Waals Equation of State Critical isotherm of a pure substance has an inflection point at the critical point. 31 Summary—system, state, property, and phase • • • • • • • • • • System: a pure substance of a fixed number of molecules: H2O. Two phases: liquid and gas. Many (thermodynamic) states, specified by two independent thermodynamic variables (properties). T,V as independent variables. Curves of constant P represent function P(T,V). A point on the left of the dome represents a state of liquid, a point on the right of the dome represents a state of gas, and a point under the dome represents a state of coexistent phases. P,V as independent variables. Curves of constant T represent function T(P,V). P,T as independent variables. Many states of coexistent phases fall on the same point on the phase boundary. Change of phase: discontinuous change of state. A single state is represented by three points on three planes. The states of coexistent phases are represented by the regions under the domes on the T-V plane and P-V plane, and by the phase boundary on the P-T plane. P and T are intensive properties. V is an extensive property. a P critical point a liquid gas 32 T Three phases Triple point liquid sublimation/cond ensation melting/freezing evaporation/cond ensation 33 https://en.wikipedia.org/wiki/Water_(data_page) 34 Liquid water is denser than ice The crystalline structure of ice is very open. Liquid water packs tighter. Ice floats on top of water http://chemistry.elmhurst.edu/vchembook/122Adensityice.html 35 http://www.wardteam.com/Blog/Preventing-Frozen-Pipes 36 37 https://commons.wikimedia.org/wiki/File:Phase_diagram_of_water.svg Phase diagram unlike that of water 38 39 The function P(T,V) 40 Project a surface in 3D to planes 41 Borgnakke and Sonntag, Fundamentals of Thermodynamics Project a surface in 3D to planes 42 Borgnakke and Sonntag, Fundamentals of Thermodynamics Phase diagram on P-V plane 43 Questions that motivate later lectures 1. 2. 3. 4. 5. What is temperature? What is a thermodynamic state? Why does a system isolated for a long time reach equilibrium? What is the molecular picture of equilibrium? Once in equilibrium, the isolated system will never get out of equilibrium. Why? 6. The phase diagrams of many pure substances look similar (i.e., coexistent phases, triple point, critical point). Why? 7. Beside TVP, what are other thermodynamic properties? 8. How do we use diagrams and tables of properties to design engines? 9. How do we invent new devices? 10. How about impure substances, such as air and saltwater? 44