Group 3A: The Boron Group
advertisement

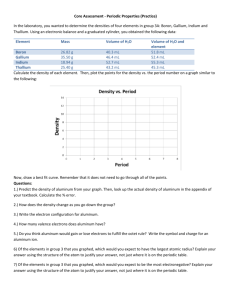
Group 3A: The Boron Group Boron Aluminum Gallium The Boron Group Boron Gallium Aluminum The Boron Group • Group 3A elements are always found combined with other elements in nature. • Based on the group number you would expect for group 3A elements to lose 3 valence electrons to form ions with 3+ charge. • Boron, Aluminum, Gallium, and Indium form such ions, but Thallium does not. • Thallium is the most metallic member of the group with properties similar to those of alkali metals. • Gallium and indium can form ions with a 1+ charge. Boron Although group 3A is named for metalloid boron, as with other groups, the lightest member is the least representative. Boron has more in common with silicon in group 4A than with the metallic members of group 3A. The main source of boron is a complex compound of boron called borax. Borax is used as a cleaning agent and as fire proof insulation. Boric acid, another compound of boron is used as a disinfectant and an eyewash. A form of boron nitride is the second hardest known material ; only diamond is harder. These materials are classified as superabrasives. Aluminum Aluminum is the most abundant metal and the third most abundant element in earth’s crust. Aluminum oxide is the major compound in bauxite. It is used as an abrasive, to strengthen ceramics, and in heat resistant fabrics. The compound aluminum sulfate, known as alum, is used in antiperspirants and to remove suspended particles during water purification. Gallium • • • • • • Gallium can literally melt in your hand. A compound of gallium and arsenic called gallium arsenide produces an electric current when it absorbs light. This property makes gallium arsenide ideal for the semiconductor chips used in light powered calculators and solar panels. Scientist are using a compound of gallium and nitrogen, gallium nitride, to develop lasers that emit blue rather than red light. Using the shorter wavelengths of blue light would triple the storage capacity of a DVD. Metical devices for detecting cancer cells could be less expensive if they use low cost, blue light lasers. Quiz!! What is the main source of Boron? Borax What is it used for? Cleaning and fireproof insulation What is the most abundant metal? Aluminum What is the compound aluminum sulfate used in? Antiperspirants What is a compound of gallium and nitrogen, and gallium and nitride used to make? Lasers that emit blue light