Unit 2: Energy
advertisement

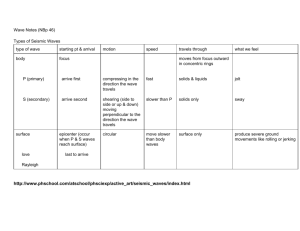
UNIT 2: ENERGY Physical Science Hobbs Bell Ringer: Friday, August 29, 2014 Calculate the speed for a car that went a distance of 125 kilometers in 2 hours time. The Nature of Energy -Energy is the ability to do work. When work is done, energy is transformed or transferred. -The SI unit of energy is the joule (J)… the same as the unit for work. Potential and Kinetic Stored energy is called potential energy The energy of motion is called kinetic energy Potential Energy/Gravitational Potential Energy -Potential energy may be stored in chemical bonds or due to the “position” of the object. -The energy of position is called “gravitational potential energy.” Kinetic Which picture shows balloons with high kinetic energy? Potential vs. Kinetic At letter “W” does the roller coaster car have high potential energy or high kinetic energy? Potential vs. Kinetic At letter “X” does the roller coaster car have high potential energy or high kinetic energy? Potential vs. Kinetic When Katniss is aiming her arrow at the deer, what kind of energy does the arrow have? Energy Transfer Potential Energy Energy Transfer Kinetic Energy GPE - Practice Calculate the GPE in the following systems: A car with a mass of 1200kg at the top of a 42m high hill GPE = mass x gravitational constant x height GPE = 1200kg x 9.8 m/s2 x 42m = 4.9 x 105 J Make sure mass is in kg and distance is in meters. GPE - Modeled A 2.3kg ball rolls 14 meters. How much gravitational potential energy does it posses? GPE - Modeled A 40kg object is lifted to a height of 50 meters. What is the GPE of the object? Kinetic Energy -The square applies only to the velocity; therefore, KE depends more on the speed or velocity than the mass. -Anything that has mass and motion will have kinetic energy; so, something as small as an atom will have KE. Kinetic Energy -Kinetic Energy is the energy of motion. -Kinetic Energy = ½ mass x velocity2 KE = ½ m x v2 KE – Practice Problems -Calculate the kinetic energy of a 1500kg car moving at the following speed 18 m/s = ½ (1500kg) x (18m/s)2 = 2.4 x 105J *Make sure the mass is in kg and the speed is in m/s* KE KE- Modeled A 2kg chair is thrown with a velocity of 8 m/s. What is the kinetic energy of the chair? KE - Modeled What is the kinetic energy of a 89kg object moving at 26 m/s? KE – A 7kg rock is thrown with a velocity of 12 m/s. What is the kinetic energy of the rock? What is the kinetic energy of a 62kg object moving at 23 m/s? Potential and Kinetic: Review Activity 1. 2. Find at least 3 pictures for each (potential and kinetic) in the magazines With the pictures you chose explain how each is either kinetic or potential. Mechanical Energy Mechanical energy is a “large-scale” combination of the kinetic and potential energy within a system; this is the type of energy we usually think of Ex: The intercom falling from the wall is a form of mechanical energy, combining both GPE (height) and KE (speed) Extension Activity 1. 2. 3. 4. 5. http://phet.colorado.edu/en/simulation/ener gy-skate-park-basics Click run now Open up bar graph and pie chart If you change the skater’s mass what happens to the energy? Design a skate park using the concepts of mechanical energy and energy conservation. 3. Bell Ringer: Tuesday, September 2, 2014 The mass of a 2013 Chevy Silverado crew cab is 4850 kg. If it was traveling down the highway at 85km/h, what is the total kinetic energy of the vehicle? Potential Energy A 56kg object is lifted to a height of 16 meters. What is the potential energy of this object? Potential Energy A 15kg squirrel climbs 12m up a tree. When he stops, what is the squirrel’s potential energy? Kinetic Energy What is the kinetic energy of a 96kg object moving 36 m/sec? Kinetic Energy The kinetic energy of a truck is calculated at 96,000J. If the truck has a mass of 50,000kg, with what velocity is it moving? Kinetic and Potential Energy Problems #7 and #10 change N to kg Forms of Energy Foldable 1. 2. 3. Fold piece of paper hot dog style 7 Sections for 7 forms of energy Each section should have: 1. 2. 3. Form of energy The definition An example of that form of energy On the back make two lists one for POTENTIAL and one for KINETIC, the forms of energy should go under a type they cannot be both. MECHANICAL, RADIANT, THERMAL, NUCLEAR, CHEMICAL, SOUND, ELECTRICAL 4. Law of Conservation of Energy • • • • Conservation of energy – does not mean saving energy The law of conservation of energy says that energy is neither created nor destroyed. So when we use energy it doesn’t disappear. We change it from one form of energy into another. 4. Bell Ringer: Wednesday, 09/03/2014 What is the kinetic energy of a 1000 kg car traveling at 20 m/s _______. a. 200,000 J b. 20,000 J c. 400,000 J d. 20,000,000 J Potential Energy: Mechanical • • • Energy stored in objects by tension. Compressed springs and stretched rubber bands are examples of stored mechanical energy. Combination of KE + PE Kinetic Energy: Radiant • • • • Electromagnetic energy that travels in transverse waves. Radiant energy includes visible light, x-rays, gamma rays and radio waves. Light is one type of radiant energy. Sunshine is radiant energy, which provides the fuel and warmth that make life on Earth possible. Kinetic Energy: Thermal • • • Thermal energy, or heat, is the vibration and movement of the atoms and molecules within substances. As an object is heated up, its atoms and molecules move and collide faster. Geothermal energy is the thermal energy in the Earth. Potential Energy: Nuclear • • • • Energy stored in the nucleus of an atom — the energy that holds the nucleus together. Very large amounts of energy can be released when the nuclei are combined or split apart. Nuclear power plants split the nuclei of uranium atoms in a process called fission. The sun combines the nuclei of hydrogen atoms in a process called fusion. Potential Energy : Chemical • • • • Chemical energy is stored in the bonds of molecules. This is a form of potential energy until the bonds are broken. Fossil fuels and biomass store chemical energy. Products that contain chemical energy include: TNT, baking soda, and a match. Kinetic Energy: Sound • • • Sound is the movement of energy through substances in longitudinal (compression/rarefaction) waves. Sound is produced when a force causes an object or substance to vibrate — the energy is transferred through the substance in a wave. Typically, the energy in sound is far less than other forms of energy. Kinetic Energy: Electrical • • Electrical energy is delivered by tiny charged particles called electrons, typically moving through a wire. Lightning is an example of electrical energy in nature, so powerful that it is not confined to a wire. Potential Energy: Gravitational • • • • Gravitational energy is energy stored in an object's height. The higher and heavier the object, the more gravitational energy is stored. When you ride a bicycle down a steep hill and pick up speed, the gravitational energy is being converted to motion energy. Hydropower is another example of gravitational energy, where the dam "piles" up water from a river into a reservoir. Kinetic Energy: Motion • • • • • Energy stored in the movement of objects. The faster they move, the more energy is stored. It takes energy to get an object moving, and energy is released when an object slows down. Wind is an example of motion energy. A dramatic example of motion is a car crash, when the car comes to a total stop and releases all its motion energy at once in an uncontrolled instant. Energy Transformations within a System Energy Transformations within a System Energy Transformations within a System Energy Transformations within a System Summarizer Half Sheet of paper What are the energy transformations within this system? Review: Energy transformations What does the law of conservation of energy state? Review: Energy Forms Review: energy forms Review: energy forms Review: energy forms Review: energy Forms Review: Energy Trans in a System REVIEW: Energy Trans in a System REVIEW: energy trans in a system 5. Bell Ringer: Thursday, 09.04.14 Name the types of energy IN ORDER that flow within this system. Heat Transfer -Vocabulary sheet -you need four different colors -if you do not have three different colors, you will draw three different shapes with your pencil/pen -Draw squares around the following key words: Heat Transfer Conduction Convection Radiation conduction rays or waves radiates high temperature bump fluid vents low temperature contact radiation solid heat transfer convection flows Heat Transfer Linking the underlined words in the same color, you may write notes on the lines between key words. Heat Transfer Thermal energy transfer is movement of thermal energy from an area of high temperature to an area of low temperature Heat Transfer Heat Transfer: Conduction Transfer of heat that happens when molecules bump into each other, usually solid object Conduction=CONTACT Have you ever? Touched a metal spoon sitting in a cup of hot coffee? What happened? Think back to what you know about metals and nonmetals. What conducts heat better, metal or nonmetal? Why? Heat Transfer: Conduction In that cup of hot water with the spoon, the following is happening: The fast-moving particles of the coffee come in contact with the slowmoving particles of the cool metal spoon. Then the slower particles in the metal spoon begin to move faster and heat is being transferred. As the particles move faster, the metal spoon gets hotter. This process is repeated until the entire spoon is hot. EXAMPLE OF CONDUCTION An ice cube in your hand (contact) Heat Transfer: Convection • • • Transfer of heat by the flow of fluids (liquids and gases) Convection = VENTS Convection has a circular pattern Have you ever? Noticed that the air near the ceiling is warmer than the air near the floor? Why do you think this happens? Warm air is less dense and rises Cold air is more dense and sinks Heat Transfer: Convection • • • • • When the water at the bottom of a pot is heated, its particles move faster, and they also move farther apart. The heated water becomes less dense. Therefore, the heated water rises in the pot. The cooler water at the top of the pot sinks. The movement of the fluids creates a circular motion of particles called convection currents EXAMPLE OF CONVECTION A radiator (vent) Heat Transfer: Radiation Heat is Transferred in rays or waves Matter is not required to transfer thermal energy Radiation = Radiates Have you ever… Place your hand really close to a light bulb that was turned on? What did you feel? Heat Transfer: Radiation All objects radiate energy and heat, even your own body. However, the radiation coming from hotter objects is more intense than that coming from cooler objects. The hotter an object, the shorter the wavelength of this radiation. EXAMPLE OF RADIATION Fire (radiates) Conduction, Convection, or Radiation Conduction, Convection, or Radiation Review What state does the following occur? Particles are in fixed a regular arrangement Review In which states? Are the particles randomly arranged and free to move? REVIEW Which sort of heat transfer… Does not require particles? REVIEW Give an example of where convection currents can occur… Summarizer: 3-2-1 HALF SLIP OF PAPER Three things you learned today Two things you kind of understand, but may still be unsure of One thing know you need to do better in this class. Bell Ringer: Wednesday, 02.12.14 A heat source is located under one end of a solid material. What process, represented in the illustration, carries heat to the other end of the block? A. convection B. radiation C. conduction D. none of the above KE The mass of a 2013 BMW i8 is 5650 kg. If it was traveling down the highway at 85km/h, what is the total kinetic energy of the vehicle? KE What is the mass of a cart that has a kinetic energy of 5,554 J and a speed of 10.98m/s? KE A 250g Nerf dart is shot from Saxon’s Nerf gun with a kinetic energy of 4.4J. What is the initial speed of the dart? PE A ball is at the top of a 40 m ramp. It has a mass of 20 kg. What is the ball’s GPE? PE A box with a mass of 12.5 sits on the floor. How high would you need to lift it for it to have a GPE of 355 J ? PE A marble is on a table 2.4 m above the ground. What is the mass of the marble if it has a gravitational potential energy of 568 J. Review HEAT TRANSFER Heat Transfer: Conduction What are the key words? Conduction is…. Heat Transfer: Convection Heat Transfer: Radiation Reading Essentials (BLUE BOOK) Chapter 6: Section 2 THERMAL ENERGY, USING HEAT on your own paper Answer questions on the sides under headings (Identify definitions, reading checks, think it over, picture this) and the questions in the section “AFTER YOU READ” Bell Ringer: Thursday, 02.13.14 What are the energy transformations within these systems (in order)? Bell Ringer: Tuesday, 02/18/2014 Name examples of each: Conduction Convection Radiation Specific Heat Capacity Specific Heat Capacity can be thought of as a measure of how much heat energy is needed to warm the substance up. You will possibly have noticed that it is easier to warm up a saucepan full of oil than it is to warm up one full of water. Specific heat capacity: SHC Specific Heat Capacity (C) of a substance is the amount of heat required to raise the temperature of 1g of the substance by 1oC (or by 1 K). SHC The next table shows how much energy it takes to heat up some different substances. The small values show that not a lot of energy is needed to produce a temperature change, whereas the large values indicate a lot more energy is needed. SHC: The Equation The amount of heat energy (q) gained or lost by a substance = mass of substance (m) X specific heat capacity (C) X change in temperature (ΔT) q = m x C x ΔT SHC Explanation: The change in temperature (ΔT) is: 75ºC - 25ºC = 50ºC Given mass, two temperatures, and a specific heat capacity, you have enough values to plug into the specific heat equation q = m x C x ΔT and plugging in your values you get q = (450 g) x (0.385 J/g ºC) x (50.0ºC) = 8700 J SHC: Effect of Mass on heat Large object Smaller object mcΔT Q is bigger = mcΔT = Q is smaller Conclusion: bigger objects need more energy to raise their temperature SHC: EFFECT of SHC on HEAT Large Specific Heat = m ΔT c Q is bigger Smaller Specific Heat = mcΔT Q is smaller 4.18 J/g·°C 0.32 J/g·°C Conclusion: bigger SHC need more energy to raise their temperature by the same amount SHC: Effect of Temp CHG on HEAT Large Temp Change = mc ΔT Q is bigger Smaller Temp change = mcΔT Q is smaller Conclusion: bigger SHC need more energy to raise their temperature by the same amount 7. Bell Ringer: Monday, 09.08.2014 A 10.0 kg block of lead is heated in the Sun from 25.0ºC to 30.0ºC. Use the table to help calculate the change in the block's thermal energy? A. 900 J B. 1300 J C. 6450 J D. 3900 J SHC: Guided How much heat is required to raise the temperature of 180.0 g of mercury by 52°C? (specific heat for mercury is 0.140 J/g°C) SHC How much energy would be needed to heat 250 grams of copper metal from a temperature of 10.0ºC to a temperature of 35.0ºC? (The specific heat of copper at 10.0ºC is 0.385 J/g ºC.) SHC Calculate the amount of heat needed to increase the temperature of 150g of water from 15oC to 30oC. The specific heat of water is 4.18 J/gºC. SHC: DO ON YOUR OWN Calculate the specific heat capacity of copper given that 204.75 J of energy raises the temperature of 15g of copper from 25o to 60o. SHC 216 J of energy is required to raise the temperature of aluminum from 15o to 35oC. Calculate the mass of aluminum. (Specific Heat Capacity of aluminum is 0.90 J/goC). SHC: Guided The temperature of a piece of Metal X with a mass of 95.4g increases from 25.0°C to 48.0°C as the metal absorbs 849 J of heat. What is the specific heat of Metal X? SHC: Guided When 435 J of heat is added to 3.4 g of olive oil at 21°C, the temperature increases to 85°C. What is the specific heat of the olive oil? SHC A piece of stainless steel with a mass of 1.55 g absorbs 141 J of heat when its temperature increases by 178°C. What is the specific heat of the stainless steel? 8. Bell Ringer: Tuesday, 09.09.2014 THURSDAY IS BLING DAY! How much energy would be needed to heat 300g of copper metal from a temperature of 10.0ºC to a temperature of 50.0ºC? (The specific heat of copper at is 0.385 J/g ºC.) SHC Approximate values in J / kg °K of the Specific Heat Capacities of some substances are: Air Aluminum Asbestos Brass Brick Concrete Cork Glass Gold Ice Iron 1000 900 840 400 750 3300 2000 600 130 2100 500 Lead 125 Mercury 14 Nylon 1700 Paraffin 2100 Platinum 135 Polythene 2200 Polystyrene 1300 Rubber 1600 Silver 235 Steel 450 Water 4200 SHC: Specific Heat of Some Common Materials Substance Specific Heat [(J/gºC)] Water Wood Carbon Glass Iron 4.184 1.760 .710 0.664 0.450 So, if the specific heat capacity of a substance is high, it requires a very large amount of energy to increase the temperature, and if it has a low specific heat capacity, the required energy will be lower. SHC Why does land heat up more quickly than sea water and how does this help to explain land and sea breezes? Specific heat capacities: Water 4200 J/kgoC Copper 400 J/kgoC Aluminium 900 J/kgoC Concrete 3300 J/kgoC Lead 126 J/kgoC SHC Why do you think that houses built of stone take a long time to warm up but once they are warm they stay warm for a long time? Specific heat capacities: Water 4200 J/kgoC Copper 400 J/kgoC Aluminium 900 J/kgoC Concrete 3300 J/kgoC Lead 126 J/kgoC SHC Should saucepans be made of material with a high or low specific heat capacity? Specific heat capacities: Water 4200 J/kgoC Copper 400 J/kgoC Aluminium 900 J/kgoC Concrete 3300 J/kgoC Lead 126 J/kgoC SHC Why is water such a good coolant? Specific heat capacities: Water 4200 J/kgoC Copper 400 J/kgoC Aluminium 900 J/kgoC Concrete 3300 J/kgoC Lead 126 J/kgoC Heat: Review for GRADE! pg.158 1. Distinguish between temperature and heat. 2. How does heat flow? 3. How does the specific heat capacity of water compare to other common substances? 4. How much heat is required to raise the temperature of 140.0 g of mercury by 58°C? (specific heat for mercury is 0.140 J/g°C) 5. Calculate the amount of heat needed to increase the temperature of 300g of water from 20oC to 50oC. The specific heat of water is 4.18 J/gºC. BONUS: A piece of stainless steel with a mass of 3.02g absorbs 203 J of heat when its temperature increases by 168°C. What is the specific heat of the stainless steel? Phases of Matter Matter has two characteristics: Matter has mass Matter takes up space All matter is made up of atoms Phases of Matter: Law of Conservation of Mass The law of conservation of mass: States of Matter Changes between states are called “phase changes”. Caused by a change of heat or pressure. More often heat. HEAT and TEMPERATURE are not the same thing. Temperature Measures the average kinetic energy of the particles in a substance. Is measured in Celsius or Kelvin. Kinetic energy is directly related to the speed of the molecules. The faster the particles/molecules are moving the higher the temperature. Heat Heat is a measurement of energy (Joules). HEAT and TEMPERATURE are not the same thing. Ex. A cold Lake Superior has more heat energy than a boiling pot of water. For our class, higher temperature means more heat. There are no shortcuts to any place worth going. – Beverly Sills 9. Bell Ringer: 09.10.14 Distinguish between HEAT and TEMPERATURE. TOMORROW is BLING DAY States of Matter Solid Liquid Gas Plasma States of Matter: Solid Particles in a solid: Are tightly packed, usually in a regular crystal lattice pattern. vibrate (jiggle) but generally do not move from place to place. More dense States of Matter: Liquid Particles in a liquid: Are close together with no regular arrangement. Vibrate, move about, and slide past each other. States of Matter: Gas Particles in a gas: Are well separated with no regular arrangement. Vibrate and move freely at high speeds. Plasma A plasma is an ionized gas, in which a certain proportion of electrons are free rather than being bound to an atom or molecule. Plasmas are by far the most common phase of matter in the universe Characteristics of Gases, Liquids and Solids gas liquid solid assumes the shape and assumes the shape of the part retains a fixed volume and volume of its container of the container which it shape particles can move past one occupies rigid - particles locked into another particles can move/slide past place one another compressible lots of free space between particles not easily compressible little free space between particles not easily compressible little free space between particles flows easily flows easily does not flow easily particles can move past one particles can move/slide past rigid - particles cannot another one another move/slide past one another Phase Change When a substance changes states it requires the input or the removal of energy or change in pressure. During a phase change the temperature does not change, but the amount of energy does. Intermolecular Forces Interactions between particles that cause them to “stick” together. Strongest in solids Weakest in gases. Strongest when particles move slowly. During a phase change IMF are either weakened or strengthened. Phase Changes: Melting – the change from solid to liquid Freezing – the change from liquid to solid Vaporization – the change from liquid to gas Evaporation – vaporization from the surface of a liquid Phase Changes Boiling – vaporization from within as well as from the surface of a liquid Condensation – the change from gas to liquid Sublimation – the change from solid to gas Deposition – the change from gas to solid Sublimation Transformation of a substance to a gas from a solid state with no liquid transition. Ex: Dry Ice does this. Deposition When a gas transforms into a solid without transitioning through a liquid state. Ex. Frost forming on windows. Where does all the energy go? During a phase change energy is added, but the temperature does not increase. The energy goes toward breaking up weak intermolecular forces between the particles. Phase Change Diagram Directions: Label the phase change of each arc. Brainstorm at least one example for each phase change and write it under each phase change. In the boxes under the phases draw a small picture of how the molecules are arranged. Phase Change Phase Change pg 502 1- 6 ON YOUR OWN PAPER, DO NOT WRITE QUESTION You learn something everyday if you pay attention – Ray LeBlond 10. Bell Ringer: 09.11.14 On which points on the graph is water increasing in temperature? Phase Change Diagram Phase Diagram Phase Change Diagrams Relates temperature and pressure or temperature and composition phase boundaries refer to the lines that identify where phase transitions occur. Phase Change Diagrams Triple point – the point on a phase diagram at which the three states of matter: gas, liquid, and solid coexist Phase Change Diagrams Critical point – the point on a phase diagram at which the substance is indistinguishable between liquid and gaseous states Phase Change Diagrams Phase Changes From.. To… Is called… And energy is… Solid Liquid Melting Absorbed Liquid Solid Freezing Released Liquid Vapor Boiling or Absorbed Vaporizati on Vapor Liquid Condensat Released ion Solid Vapor Sublimatio Absorbed n Vapor Solid Deposition Released Phase Change Diagram A B What is the melting point of this substance? If the temperature is increased at point A to 400 º C, what would the phase of matter be? If the pressure of the matter at point B is increased to 70 atm, what would be the phase of matter? What is work? In science, the word work has a different meaning than you may be familiar with. The scientific definition of work is: using a force to move an object a distance (when both the force and the motion of the object are in the same direction.) Work or Not Work? A teacher lecturing to the class A mouse pushing a piece of cheese with its nose across the floor. Work or Not Work? Work or Not Work? A scientist delivers a speech to an audience of his peers. A body builder lifts 350 pounds above his head. A mother carries her baby from room to room. A baseball player hits a baseball to outer field. A mother carries her child up the stairs of the Eiffel Tower. Work or Not Work? A scientist delivers a speech to an audience of his peers. No A body builder lifts 350 pounds above his head. Yes A mother carries her baby from room to room. No A baseball player hits a baseball to outer field. Yes A mother carries her child up the stairs of the Eiffel Tower. Yes Work Work - is the transfer of energy that occurs when a forces makes an object move. Work Work = Force x Distance The unit of force is newtons The unit of distance is meters The unit of work is newton-meters One newton-meter is equal to one joule So, the unit of work is a joule W=(F)(D) Work = Force x Distance Calculate: If a man pushes a concrete block 10 meters with a force of 20 N, how much work has he done? W=(F)(D) Work = Force x Distance Calculate: If a man pushes a concrete block 10 meters with a force of 20 N, how much work has he done? 200 joules (W = 20N x 10m) Power Power is the rate at which work is done. Power =Work*/Time *(force x distance) The unit of power is the watt. CHECKPOINT 1 1. Blayne pushes a cart 40m down the hallway with a force of 10N, how much work is being done? CHECKPOINT 1 2. 10, 000J of work is done to move a car 25m. How much force was applied? Checkpoint 3 3. How far would a paper airplane go if it had a force of 15N and 12J of work was done? 11. Bell Ringer: 09.12.14 WEEKLY REVIEW 1 DUE TODAY A If the pressure of the matter at point A is increased to 75 atm, what would be the phase of matter? What would be the phase change(s)? Simple Machines There are six simple machines Lever Wheel and Axle Pulley Inclined Wedge Screw Plane Simple Machines A machine is a device that helps make work easier to perform by accomplishing one or more of the following functions: transferring a force from one place to another changing the direction of a force increasing the magnitude of a force, or increasing the distance or speed of a force Simple Machines: Lever A lever is a rigid bar that rotates around a fixed point called the fulcrum. The bar may be either straight or curved. In use, a lever has both an effort (or applied) force and a load (resistant force). Simple Machines: Changes the direction of the force Multiplies effort force Magnifies speed and distance Ex: seesaw, crowbar, scissors st 1 Class Lever Simple Machine: 1st Class Lever A first-class lever always changes the direction of force (I.e. a downward effort force on the lever results in an upward movement of the resistance force). Simple Machines: Multiply effort force Mechanical advantage is always greater than 1. Ex: bottle opener, boat oars, wheel barrow nd 2 Class Lever Simple Machine: 2nd Class Lever A second-class lever does not change the direction of force. When the fulcrum is located closer to the load than to the effort force, an increase in force (mechanical advantage) results. Simple Machine: Magnifies speed and distance Mechanical Advantage always less than 1 Ex: baseball bat, golf club, broom, shovel rd 3 Class Lever Simple Machine: 3rd Class Lever A third-class lever does not change the direction of force; thirdclass levers always produce a gain in speed and distance and a corresponding decrease in force. Simple Machine: Wheel and Axle The wheel and axle is a simple machine consisting of a large wheel rigidly secured to a smaller wheel or shaft, called an axle. When either the wheel or axle turns, the other part also turns. One full revolution of either part causes one full revolution of the other part. EX: doorknob, pencil sharpener, screwdriver, steering wheel Simple Machine: Inclined Plane An inclined plane is an even sloping surface. The inclined plane makes it easier to move a weight from a lower to higher elevation. Simple Machine: Inclined Plane The mechanical advantage of an inclined plane is equal to the length of the slope divided by the height of the inclined plane. While the inclined plane produces a mechanical advantage, it does so by increasing the distance through which the force must move. Simple Machine: Pulley A pulley consists of a grooved wheel that turns freely in a frame called a block. A pulley can be used to simply change the direction of a force or to gain a mechanical advantage, depending on how the pulley is arranged. Simple Machine: Pulley A pulley is said to be a fixed pulley if it does not rise or fall with the load being moved. A fixed pulley changes the direction of a force; however, it does not create a mechanical advantage. A moveable pulley rises and falls with the load that is being moved. A single moveable pulley creates a mechanical advantage; however, it does not change the direction of a force. The mechanical advantage of a moveable pulley is equal to the number of ropes that support the moveable pulley. Simple Machine: Wedge The wedge is a modification of the inclined plane. Wedges are used as either separating or holding devices. A wedge can either be composed of one or two inclined planes. A double wedge can be thought of as two inclined planes joined together with their sloping surfaces outward. Simple Machine: Screw The screw is also a modified version of the inclined plane. Screw is an inclined plane wound around a central cylinder. While this may be somewhat difficult to visualize, it may help to think of the threads of the screw as a type of circular ramp (or inclined plane). 12. Bell Ringer: Monday, 09.15. 2014 How much work is done on a 20 N block that is lifted 4 meters off the ground by a pulley? THURSDAY is SENIOR CITIZENS DAY time to RAID your Grandparent’s or Great Grandparent’s closet and bring out the suspenders, bowties, mumus, and hair rollers in support of the TROJANS! Work: Review How much work is done on a 10 N block that is lifted 5 meters off the ground by a pulley? Work: Review Malone lifts her book bag 1.5 meters. If the weight of the bag is 12 N, how much work did she do? Work: Review Sayer lifts a 45 N bag of mulch 1.2 meters and carry it a distance of 10 meters to the garden. How much work was done? Mechanical Advantage It is useful to think about a machine in terms of the input force (the force YOU apply) and the output force (the force which applied by the machine) Input (you, effort) Output (resistance, load) Mechanical Advantage MA MA= Fr(output) Fe(input) F=force; e=effort; r=resistance **use with newtons MA MA= de(distance effort) dr (distance resistance) d=distance; e=effort; r=resistance **use with distance (meters) Mechanical Advantage: Guided Jackson lifts a load 10cm with a pulley system, 20 cm of string had to be pulled. What is the mechanical advantage? MA: Guided Kent applied 20N of force to turn an ice cream freezer crank. The crank’s resistance was 60 N. What was the mechanical advantage of the crank? MA: Guided A simple machine uses 20 N of input force to lift a 80 N chair. What is the mechanical advantage of this simple machine? CHECKPOINT 2 What is the mechanical advantage of a lever that has an input arm of 3 meters and an output arm of 2 meters? CHECKPOINT 2 A rake is held so that its input arm is 0.5 and its output arm is 1.0 meters. What is the mechanical advantage of the rake? MA Suppose Royce needs to remove a nail from a board by using a claw hammer. If the effort length for a claw hammer is 11.0 cm and the resistance length is 2.0 cm. What is the mechanical advantage? MA A pulley is used to raise a heavy crate. The pulley is such that an input force of 223 N is needed to provide an output force of 1784 N. What is the mechanical advantage of this pulley? CHECKPOINT 3 What is the item? What class of lever is the item? CHECKPOINT 3 What is the item? What class of lever is the item? CHECKPOINT 3 What is the item? What class of lever is the item? CHECKPOINT 4 Simple Machines What are the purposes of simple machines? Simple Machines Identify some simple machines in your daily life (not examples used today). W F Fr D MA de MA dr Fe 13. Bell Ringer: 09.16.14 YOU HAVE A NEW SEAT CHECK THE DESKS!!! Kent applied 30N of force to turn an ice cream freezer crank. The crank’s resistance was 80 N. What was the mechanical advantage of the crank? THURSDAY is SENIOR CITIZENS DAY time to RAID your Grandparent’s or Great Grandparent’s closet and bring out the suspenders, bowties, mumus, and hair rollers in support of the TROJANS! Waves A. Wave is a repeating disturbance or movement that transfers energy through matter or space. 1. A wave will travel only as long as it has energy to carry. Waves Mechanical Waves-require a medium in order to transport their energy from one location to another. Electromagnetic waves-are transverse waves that travel without a medium. So they travel through empty space. Waves A wave is a form of energy transfer from one point of space (medium) to the other. Properties of Waves G. Electromagnetic waves are transverse waves that travel without a medium. So they travel through empty space. 1. Electromagnetic waves travel as vibrations in electrical and magnetic fields. Waves 2.Electromagnetic spectrum - name for the range of waves when placed in order of increasing frequency. Waves 1. The two types of mechanical waves are transverse waves and compressional waves. 2. Transverse wave matter in the medium moves back and forth at right angles to the direction that the wave travels. Waves 3. In a compression wave, matter in the medium moves back and forth along the same direction that the wave travels. 4. Compressional waves are also called longitudinal waves. Waves 5. Sound waves are compressional waves. Compressions travel through the air to make a wave. Waves D. Seismic waves are a combination of compressional and transverse waves. They can travel through Earth and along Earth’s surface. Waves A. The transverse wave has alternating high points, called crests, and low points, called troughs. 1. A compressional wave has no crests and troughs, but more dense regions called compressions. 3. The less-dense regions of a compressional wave is called a rarefactions. Properties of Waves B. Wavelength - is the distance between one point on a wave and the nearest point just like it. 1. In a transverse wave, the wavelength is distance from crest to crest or trough to trough. 2. A wavelength in a compressional wave is the distance between two neighboring compressions or two neighboring rarefactions. Properties of Waves frequency – the number of full vibrations each point of the wave completes in 1 s period – time it takes the wave to travel one wavelength; Properties of Waves The speed of a wave depends on the medium it is traveling through. 1. Sounds waves usually travel faster in liquids and solids than they do in gases. 2. Sound waves usually travel faster in a material if the temperature of the material is increased. Properties of Waves Wave speed – the rate at which the wave travels through a given medium; v = fλ, where v is wave speed; f – frequency; and λ – wavelength; Ø here wave speed is measured in m/s, frequency - in Hz, and wavelength – in meters; Properties of Waves F. Amplitude -is related to the energy carried by a wave. 1. The greater the wave’s amplitude is, the more energy the wave carries. 2. For a compressional wave with high amplitude, coils will be closer together in compressions and farther apart during rarefactions. Properties of Waves 3. For a compressional wave with low amplitude, coils will be farther apart in compressions and closer together during rarefactions. 4. With transverse waves, a tall ocean wave has a greater amplitude than a short ocean wave. 5. The amplitude of a transverse wave is the distance from the crest or trough of the wave to the rest position of the medium. Properties of Waves Behavior of Waves A. Reflection occurs when a wave strikes an object bounces off of it. This can occur with sound, water, and light waves. 1. According to the law of reflection, the angle of incidence is equal to the angle of reflection. Behavior of Waves B. Refraction- is the bending of a wave caused by a change in its speed as it moves from one medium to another. The greater the change in speed is, the more the wave bends. Behavior of Waves C. Diffraction occurs when an object causes a wave to change direction and bend around it. 1. When an obstacle is smaller than the wavelength, the waves bend around it. If the obstacle is larger than the wavelength, the waves do not diffract as much. Behavior of Waves 2. Diffraction affects radio’s reception. AM radio waves have longer wavelengths than FM radio waves do. AM radio waves are capable of diffracting around bigger obstacles, short FM waves do not diffract as much. Behavior of Waves D. Interference - the process where two or more waves overlap and combine to form a new wave. 1. In constructive interference, the waves add together. a. This happens when the crests of two or more transverse waves arrive at the same place at the same time and overlap. The amplitude of the new wave will equal the sum of the amplitudes of the original waves. 2. Destructive interference, the waves subtract from each other as they overlap. a. This happens when the crests of one transverse wave meets the troughs of transverse wave. The amplitude of the new wave is the difference between the amplitudes of the waves that overlapped. Behavior of Waves Behavior of Waves E. Resonance - the process by which an object is made to vibrate by absorbing energy at its natural frequencies. 15. Bell Ringer: Friday, 09.19.2014 Sound travels in a ____ wave. a. mechanical c. surface b. electromagnetic d. inverted Bell Ringer: Monday, 02.24.14 A 10.0 kg block of copper is heated in the Sun from 25.0ºC to 30.0ºC. Use the table to help calculate the change in the block's thermal energy? Wave Speed v = fλ, where v is wave speed; f – frequency; and λ – wavelength; Ø here wave speed is measured in m/s, frequency - in Hz, and wavelength – in meters; Wave Speed Wave has a frequency of 4 Hz and a wavelength of 1.6 meters. What is the speed of the wave? Wave Speed Wave has a frequency of 8 Hz and a wavelength of 2.4 meters. What is the speed of the wave? Wave Speed The speed of sound in air is 280 m/s. If the frequency of Middle D is 264 Hz, what is its wavelength? Wave Speed The wake of a boat has a speed of 5 m/s and a wavelength of 2.3m. What is the frequency? Bell Ringer: Wednesday, 02.26.14 What is another word for compressional wave? 17. Bell Ringer: Monday, 09.22.14 What is the wavelength of a wave with a frequency of 652 Hz traveling at 26 m/s? THURSDAY IS HAWAIIAN DAY!!! Review The wake of a boat has a speed of 4 m/s and a wavelength of 2.2m. What is the frequency? Review Wave has a frequency of 9 Hz and a wavelength of 3.2 meters. What is the speed of the wave? Nuclear Energy CHAPTER 9.2 Nuclear Energy Power plants use heat to produce electricity. Nuclear energy produces electricity from heat through a process called fission. Nuclear power plants use the heat produced by fission of certain atoms. Nuclear Energy Fission of U-235 splits nucleus in two pieces Uranium-235 releases neutrons for chain reaction Nuclear fission chain reaction releases energy in the form of heat Nuclear Energy - Nuclear Reactor device built to sustain a controlled nuclear fission chain reaction Main Components of Nuclear Reactor: reactor vessel tubes of uranium control rods - containment structure Nuclear Energy Fission occurs in the reactor vessel. Heat is produced. The heat is used to heat water to create steam. The steam is used to turn the turbine in the generator to produce electricity. The steam is cooled in the condenser to return to the liquid phase. Nuclear Energy Advantages Low cost predictable power at a stable price of production Do not emit harmful gases Disadvantages Radioactive waste due to nuclear reactors which leaks radiation contents Uranium – Non-renewable resource Nuclear Energy A TLD measures ionizing radiation Measures amount of visible light emitted from a crystal in the detector when the crystal is heated. The amount of light emitted is dependent upon the radiation exposure. Nuclear Energy Nuclear Energy Nuclear Energy Nuclear Energy Nuclear Energy Nuclear Energy Nuclear Energy Nuclear Energy Extra Practice Pg 835 Chp 4 - 38, 39, 40, 50 Pg 835 Chp 5 – 51, 52 Pg 836 Chp 6 – 64, 65, 66, 67 18. Bell Ringer: Tuesday, 09. 23. 14 Identify the chain reaction… QUIZ TODAY: over work and simple machines, waves, nuclear energy THURSDAY IS HAWAIIAN DAY!!! 19. Bell Ringer: 09.24.14 What is the wavelength of a wave with a frequency of 652 Hz traveling at 26 m/s? **Reminder – BELL RINGERS are DUE TODAY!!** TOMORROW IS HAWAIIAN DAY!!! TEST: UNIT 2 1 person per table Cell phones OFF and on front file cabinet Need: pencil, scratch paper, calculator Provided: Scantron, Test AFTER TEST: WORK ON Constructed RESPONSE CHOOSE 4 (need own paper) Can have cell phones after finished with Constructed Response