Hepatotoxicity Of Nonsteroidal Anti-inflammatory
advertisement

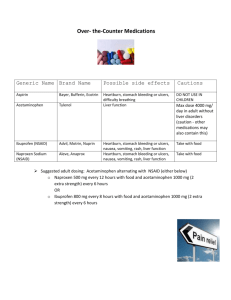
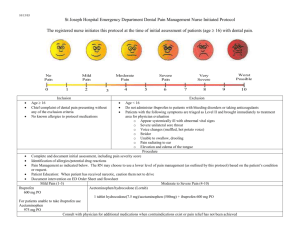
Hepatotoxicity of nonsteroidal anti-inflammatory drugs (NSAIDs) in mice Mindi Clegg Advisor: Dr. Brown Analyzing ALT levels Hepatotoxicity is one of the common side effects of nonsteroidal antiinflammatory drugs. Individual NSAIDs differ in the incidence, severity, and type of liver damage they produce (Wade et al., 1997, 546). Symptoms of hepatotoxicity can range from mild elevation in serum aminotransferases to severe hepatocellular injury (Wade et al., 1997, 546). Drug induced liver damage is the most common cause for drugs to be withdrawn from the market (Obach et al., 2008, 1814). Studies have shown that acetaminophen forms a reactive metabolite (NAPQI) that is an oxidizing agent that depletes the cell of glutathione (Gibson et al., 1996, 581). The biochemical functions of glutathione are recognized in maintaining cellular protein sulfhydryl groups and in detoxifying substances including metals, hydrogen peroxide, and oxygen radicals (Kuralay et al., 1998, 224). A depletion of glutathione has been used to evaluate toxicity, because it is involved in the response to toxins. The second assay used in this experiment was the alanine aminotransferases (ALT) assay. ALTs are normally found in large concentrations in the liver. The ALT blood test is typically used to detect liver damage, because it is released into the bloodstream due to liver injury. ALT values are often compared to the results of other tests to determine which form of liver disease is present (Abboud and Kaplowitz, 2007, 278-280). This research attempted to determine if other NSAIDs, such as naproxen and ibuprofen, have the same hepatotoxic effects on mice livers as acetaminophen. Mice were used as the study organism, because previous experiments had used them and their metabolism is similar to that of humans. The hypothesis tested in this experiment is that the hepatotoxicity of naproxen and ibuprofen will be less than that of acetaminophen, since naproxen and ibuprofen are metabolized by different enzymes in the liver. Glutathione levels and ALT levels were analyzed to determine the amount of liver damage. Dosing of mice Materials and methods Experiments were performed with 12 male mice, which were divided into four groups with three mice in each group. Mice in the same testing group were housed together in Dr. Lustofin’s office and kept on a natural light cycle during the month of February. Mice were moved to individual cages with no food or bedding and fasted 2 hours before dosing. Group 1 (control) received 200 mg of peanut butter. Group 2 received 200 mg of peanut butter with 4 mg of acetaminophen. Group 3 received 200 mg of peanut butter with 3.2 mg of ibuprofen. Group 4 received 200 mg of peanut butter with 1.6 mg of naproxen. These doses were calculated using the weight of the mouse and the half-life of the drug. Mice were dosed daily for ten days. Methods After sacrificing the mice, whole blood samples were collected. The samples were centrifuged and the serum was collected. The assay protocol listed in the Bioo Scientific Instruction Manual for the ALT Assay Kit was followed. ALT Levels in Mice Serum 200 180 160 140 120 100 80 60 40 20 0 ALT (units/L) Introduction 167.490 Control 189.598 Acetaminophen 108.716 52.441 Ibuprofen Naproxen Figure 1. Average ALT (units/L) levels in mice serum for each testing group. Results Statistically the only difference found in the ALT test was between the control and the naproxen group. The acetaminophen and ibuprofen were not statistically different from the control. Analyzing glutathione levels Methods After sacrificing the mice, livers were dissected and stored in a -80o freezer. Glutathione (GSH) level analysis was conducted to determine the concentration of GSH in the mice livers (Brown, 2009, 1-10). When GSH is converted to GSSG using DTNB, TNB is formed. A set amount of GSSG-reductase was added to the samples to measure the formation of TNB. The rate of TNB production was used as the measure of GSH present. The absorbance of TNB was measured at 412 nm. Figure 2. Shows the chemical process of converting GSH to GSSG producing TNB, which was used as a measure of GSH. Concentration of GSH in Mice Livers 5 Acknowledgements 4 [GSH] in µM I would like to thank Dr. Brown for being my capstone advisor and my capstone classmates for their support and feedback. I would like to thank Dr. Lustofin for letting me use her office to house my mice. I would also like to thank the Marietta College Biology Department for their funding and the Investigative Studies for their supplies grant to purchase the ALT Assay Kit. Finally, I would like to thank my friends and family for their encouragement during this experiment. 3 2 1 0 3.503 2.517 3.997 3.830 Control Acetaminophen Ibuprofen Naproxen Figure 3. Average concentration of GSH in mice livers for each testing group. Results The acetaminophen did cause a statistically significant decrease in the GSH levels in group 2. The GSH levels in the other test group were not statistically different from the control. Conclusions This research attempted to determine if other NSAIDs, such as naproxen and ibuprofen, have the same hepatotoxic effects on mice livers as acetaminophen. It was hypothesized that the hepatotoxicity of naproxen and ibuprofen would be less than that of acetaminophen. Although GSH levels in the acetaminophen testing group were depleted, the ALT tests show that no significant liver damage occurred, so the hypothesis was not supported. In the ALT experiment, liver damage was not observed for the dosed groups of mice. The naproxen was significantly lower than the control. However, the group that was dosed with acetaminophen, did show elevated ALT levels in the blood serum, but levels were not statistically significant. In the glutathione experiment, liver damage was observed in the group dosed with acetaminophen. The GSH levels in the livers of the mice dosed with acetaminophen were depleted. The groups dosed with ibuprofen and naproxen did not show depleted levels of GSH present. Research was not found that directly compared the three drugs tested in this experiment. However numerous amounts of research showed that a similar dose of acetaminophen caused liver damage in mice (Agarwal et al., 2010, 8). Liver damage due to acetaminophen has become more common, because it is a frequently used anti-inflammatory drug (Gibson et al., 1996, 580). It may be possible to reduce the risk of drug induced liver damage by using other NSAIDs to treat patients. The use of these other NSAIDs would still reduce the pain and inflammation in the patient, but would not cause the same degree of damage to the liver (Wade et al., 1997, 547). It would be beneficial to repeat this experiment with a larger sample size in order to determine if the ibuprofen and naproxen damage the liver less than acetaminophen. Different doses may need to be tested in order to see distinguishable results. Literature Cited Abboud G, Kaplowitz N. (2007) Drug-Induced Liver Injury. Drug Safety. 30(3):277-294. Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer K, James LP, Hinson JA (2010) Acetaminophen-Induced Hepatotoxicity in Mice Occurs with Inhibition of Activity and Nitration of Mitochondrial Manganese Superoxide Dismutase. American Society for Pharmacology and Experimental Therapeutics. 1-34. Brown D. (2009) Measuring Glutathione and Glutathione Disulfide Levels. Toxicology Lab Manual. Marietta College: 38-42. Gibson JD, Pumford NR, Samokyszyn VM, Hinson JA. (1996) Mechanism of Acetaminophen-Induced Hepatotoxicity: Covalent Binding versus Oxidative Stress. Chemical Research in Toxicology. 9:580-585. Kuralay F, Akarca US, Ozutemiz AO, Kutay F, Batur Y. (1998) Possible Role of Glutathione in Prevention of Acetaminophen-Induced Hepatotoxicity Enhanced by Fish Oil in Male Wistar Rats. Journal of Toxicology and Environmental Health. 53:223-229. Obach RS, Kalgutkar AS, Soglia JR, and Zhao SX. (2008) Can In Vitro MetabolismDependent Covalent Binding Data in Liver Microsomes Distinguish Hepatotoxic from Nonhepatotoxic Drugs? An Analysis of 18 Drugs with Consideration of Intrinsic Clearance and Daily Dose. Chemical Research in Toxicology. 21:1814-1822. Wade LT, Kenna G, Caldwell J. (1997) Immunochemical Identification of Mouse Hepatic Protein Adducts Derived from the Nonsteroidal Anti-Inflammatory Drugs Diclofenac, Sulindac, and Ibuprofen. Chemical Research in Toxicology. 10:546-555.