Document
advertisement

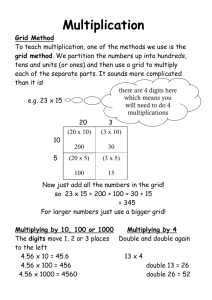
Main Grid 1/17/12 • • • • No Drill Any questions re: Midterm Review #2? Can keep review sheet until tomorrow. Check your folders for 1st midterm review and other work. Main Grid Chemistry Midterm Review Day #7 Topics Covered • • • • • • • • Chapter 1 (Measurements) Chapter 2 (Energy & Matter) Chapter 3 (Atomic Structure) Chapter 4 (Electron Configurations) Chapter 5 (The Periodic Table) Chapter 6 (Groups of Elements) Chapter 7 (Chemical Bonding/Formulas) Chapter 8 (Shapes of Molecules) Main Grid Objectives • For a complete listing of Midterm objectives, see the Midterm County Assessment Teacher’s Guide. Main Grid Agenda • Review Game 2 Main Grid 4 Categories (7 ques. ea.) • • • • Scientific Investigation Chemical Formulas Atomic Structure and Periodicity Misc. Main Grid Game Groups • • • • • Count off 1 - 7. Remember your number! The 1st group of 7 = Group 1 The 2nd group of 7 = Group 2 The 3rd group of 7 = Group 3 The 4th group of 7 = Group 4 Get in your groups and get ready to play Main Grid Game Rules • Rotate through group starting with 1 • Not allowed to help your group member • Can use periodic table, ion sheet, electronegativity sheet and calculator Main Grid Scientific Investigation Which of the following is a chemical change? A – melting wax B – boiling water C – painting black D – burning wood Main Grid Scientific Investigation • • • • • Which of these is not an SI base unit? A - Kg B-s C-L D-K Main Grid Scientific Investigation • What is the name of the lab equipment shown? • A - Watch glass • B - Crucible • C - Beaker • D - Evaporating dish Main Grid Scientific Investigation Which of the following is a homogenous mixture? A – carbon dioxide B – purified air C – a block of lead D – ice water Main Grid Scientific Investigation • How many liters are equivalent to five milliliters? A - 0.005 L B - 0.05 L C - 500 L D - 5000 L Main Grid Scientific Investigation Trial Measured Volume (L) 1 5.20 2 5.20 3 5.19 4 5.20 5 5.20 The following data was collected. The volume of the gas is known to be 2.20 L. This data reflects — A - low precision and low accuracy B - low precision and high accuracy C - low accuracy and high precision D - high accuracy and high precision Main Grid Scientific Investigation • Which of the following best describes why an experiment should be repeated? A - To organize the data B - To produce a variety of results C - To include another variable D - To verify the observed results Main Grid Chemical Formulas • The correct name for P2O5 is — A - phosphorus (V) pentoxide B - phosphorus oxide C - phosphorus (II) oxide D - diphosphorus pentoxide Main Grid Chemical Formulas • The correct formula for copper (I) bromide is — A - CuBr B - CuBr2 C - Cu2Br D - Cu2Br3 Main Grid Chemical Formulas • What type of bonds are present in (NH4)2S? A – Ionic B – Covalent C – Ionic & Covalent D – There are no bonds Main Grid Chemical Formulas • What property does CF4 most likely not have? A – High boiling point B – Soft and brittle C – Low conductivity D – Sharing of electrons Main Grid Chemical Formulas • How many atoms does (NH4)2SO4 have? A - 12 B - 18 C - 15 D - 10 Main Grid Chemical Formulas • What is the shape of CH4? A – linear B – trigonal planar C – tetrahedral D – pyramidal Main Grid Chemical Formulas • The correct name for PbSO3 is — A – Lead Sulfite B – Lead (II) Sulfite C – Lead (IV) Sulfate D – Lead Sulfur Trioxide Main Grid Atomic Structure and Periodicity • How many valence electrons does a neutral atom of silicon have? A- 3 B- 4 C- 5 D- 6 Main Grid Atomic Structure and Periodicity • A scientist has found the following isotope of oxygen: 19 O 8 How many neutrons are present in this isotope? A- 8 B - 11 C - 19 D - 27 Main Grid Atomic Structure and Periodicity • The elements that are characterized as being highly reactive — A - alkali metals B - alkaline earth metals C - halogens D - lanthanoids Main Grid Atomic Structure and Periodicity • The net charge on an aluminum ion is 3+ because there are — A - 10 protons and 13 electrons in the atom B - 13 protons and 10 neutrons in the nucleus C - 10 neutrons and 13 electrons in the atom D - 13 protons and 10 electrons in the atom Main Grid Atomic Structure and Periodicity When an atom moves from excited to ground state — A - it moves faster B - the light emitted by the atom is a type of electromagnetic radiation C - the Doppler effect shows a shift in wavelength for H-atom light D – it absorbs energy Main Grid Atomic Structure and Periodicity • What is the main similarity among elements in group 2? A - Atomic radius B - Chemical properties C - Mass number D- Boiling point Main Grid Atomic Structure and Periodicity • Which of the following requires the least energy to remove an electron? A - Br B-K C - Al D - Na Main Grid Misc. • What is the molecular geometry of carbon disulfide? A – linear and nonpolar B – bent and polar C – trigonal planar and nonpolar D – pyramidal and polar Main Grid Misc. • Which of the following is a nonpolar molecule? A – HCl B – H 2O C – CCl4 D – CH3F Main Grid Misc. Who determined that an atom is made up of mostly empty space using gold foil? A - Bohr B - Millikan C - Moseley D - Rutherford Main Grid Misc. • Which of the following best describes sublimation? A - A solid melting to a liquid B - A solid melting to a liquid, which then evaporates C - The movement of gaseous particles so that they fill the space they occupy D - A solid forming a gas Main Grid Misc. What element is represented by [He]2s22p6 ? A – Ne B – Ca C – Ar D – Li Main Grid Misc. Convert Convert 2.8 x 105 centimeters (cm) to kilometers (km). A – 20.8 km B – 2.8 km C – 0.28 km D – 0.028 km Main Grid Misc. Dumbbell shaped orbital are called: A – s-orbitals B – p-orbitals C – d-orbitals D – f-orbitals Main Grid