Diffusion & Osmosis Lab: High School Biology Experiment
advertisement

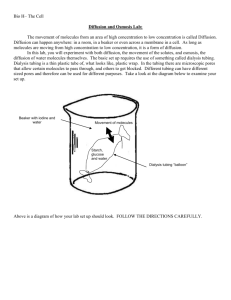
Bio H– The Cell Diffusion and Osmosis Lab: The movement of molecules from an area of high concentration to low concentration is called Diffusion. Diffusion can happen anywhere: in a room, in a beaker or even across a membrane in a cell. As long as molecules are moving from high concentration to low concentration, it is a form of diffusion. In this lab, you will experiment with both diffusion, the movement of the solutes, and osmosis, the diffusion of water molecules themselves. The basic set up requires the use of something called dialysis tubing. Dialysis tubing is a thin plastic tube of, what looks like, plastic wrap. In the tubing there are microscopic pores that allow certain molecules to pass through, and others to get blocked. Different tubing can have different sized pores and therefore can be used for different purposes. Take a look at the diagram below to examine your set up. Beaker with iodine and water Movement of molecules Starch, glucose and water Dialysis tubing “balloon” Above is a diagram of how your lab set up should look. FOLLOW THE DIRECTIONS CAREFULLY. Bio H– The Cell Part I: Diffusion of Starch and Glucose 1. Make sure you have everything from the list below at your station. If you are missing anything, let your teacher know. a. 1 empty beaker b. 1 dropper bottle of iodine c. 1 package of Glucose test strips d. 2 pieces of string e. 1 long neck funnel f. 1 10mL graduated cylinder 2. Bring your empty beaker up to the sink in the back and fill it with 250mL of tap water. 3. Add 30 drops of iodine to your beaker of water. This should be enough to make it a nice yellowy/orange. 4. Measure your iodine/water beaker with a glucose test strip. Record the initial color of the beaker solution and glucose test results on the data sheet. Making the balloon: 1. Go to the front of the room to pick up 1 dialysis tube. They have been presoaked for you to save time. 2. Fold up one end of the tube about ½ in and tie it with one piece of string as demonstrated at the beginning of class. 3. Rub your fingers together on the other end of the tube to open it up and place the long neck of the funnel into the tube. 4. Send another group member up front to get 7mL of starch/glucose solution with the graduated cylinder. 5. Slowly pour the starch/glucose solution into the funnel to fill the balloon. 6. Gently squeeze out the air. 7. Leaving as much room as possible (but no air) fold the other end of the balloon over ½ inch and tie it off with the remaining string. 8. Dip your balloon into the beaker of water to wet it and take it immediately over to the scale up front to weigh it. Make sure the scale is set to “zero” before weighing. Experiment: 1. Place your balloon into the beaker of water and iodine and record your initial weight on your data sheet. 2. You may work on part II while you wait for 15 minutes. 3. After 15 minutes, reweigh your balloon. 4. Test the solution in the beaker again for glucose. 5. Record all your observations and results on the data sheet. Clean up: 1. Throw out your balloon. 2. Empty your beaker into the sink IN THE BACK and rinse your beaker. 3. With a WET PAPER TOWEL, wipe up any drips or spills at your station. 4. Dry your station. 5. Make sure your station is properly set up as it was in the beginning Bio H– The Cell Data part I: Initial color of the iodine/water mixture: ___________________ Final color of the iodine/water mixture: ___________________ Was there glucose in the iodine/water mixture at the beginning? ______________ Was there glucose in the iodine/water mixture at the end? ___________________ Initial Mass of balloon: _____________ Final Mass of balloon: ______________ Initial color of balloon: _________________ Final color of balloon: _________________ Part II: Using Osmosis to determine the concentration of a solution Students in a class employed a similar technique to identify the concentration of a solution of sucrose. Using the same set up that you did in part I, they filled six dialysis balloons with a sucrose solution of unknown concentration. They then placed each of the six balloons into a beaker containing sucrose solution of six different known concentrations. The six known concentrations are listed in the table below. They weighed each balloon prior to placing them into their respective beakers and then allowed the “mystery” balloons to soak for 15 minutes. At the end of the 15 minutes they weighed the balloons again. All of their data is presented below. Weight of balloons in each of the following beakers Solution B (in beaker) 0.0M 0.2M 0.4M 0.6M 0.8M 1.0M Initial Final 9.4 g 10.6g 9.6g 10.2g 9.5g 9.8g 9.6g 9.3g 9.5g 8.9g 9.4g 8.5g % change in weight 1. Calculate the percent change in weight for each balloon. Use +/- to indicate whether the weight increased or decreased. 2. Graph the percent change in weight. Include all appropriate parts of a graph. Bio H– The Cell IN YOUR LAB JOURNALS Prelab – Read ENTIRE LAB including procedure and questions (you should know what we expect you to get out of this lab before diving in) Purpose: what were the goals of this lab (each part) Variables: indentify dependent and independent variables for each part Introduction: any background information on diffusion or these molecules needed to understand this lab PLUS a brief description, in your own words, of how the experiment will be done. Procedure: summarize in your words so you know what you did (a couple of sentences, not step by step) Data: All data tables, calculations and graphs Conclusion: What does your data tell you? Why is this significant? How does this relate to real cells? Scientific support for the statements you have made. Also consider the discussion questions below Discussion questions part I: 1. Which molecules in part I were able to permeate the dialysis membrane? Explain how you were able to tell. 2. What criteria determine whether molecules travel through the dialysis tubing? How does this differ from a REAL cell? 3. How does what you know about these molecules provide an explanation for why some moved and other didn’t. Discussion questions part II: 1. Why is it important to look at the percent change as opposed to just the simple change in weight? 2. Define isotonic point: a. How would this appear on a graph that looks at percent change in mass? 3. What is the concentration of the mystery solution? Explain. 4. Which balloon(s) experienced the greatest rate of diffusion? a. What relationship do you see between the rate of diffusion and the steepness of the concentration gradient? (in other words, does how does rate of diffusion change as the difference in concentration between the balloon and the beaker increases?) 5. To do this experiment the dialysis tubing must be semi-permeable. It allows water to pass, but not the sucrose used to make the sugar solution. Explain how our results might have been different if both sucrose and water were able to move. (address the direction of both molecules as well as the overall effect on the weight change)