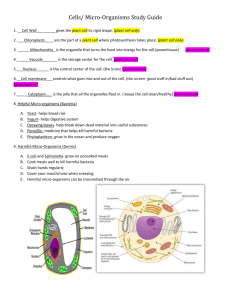
Poster
advertisement
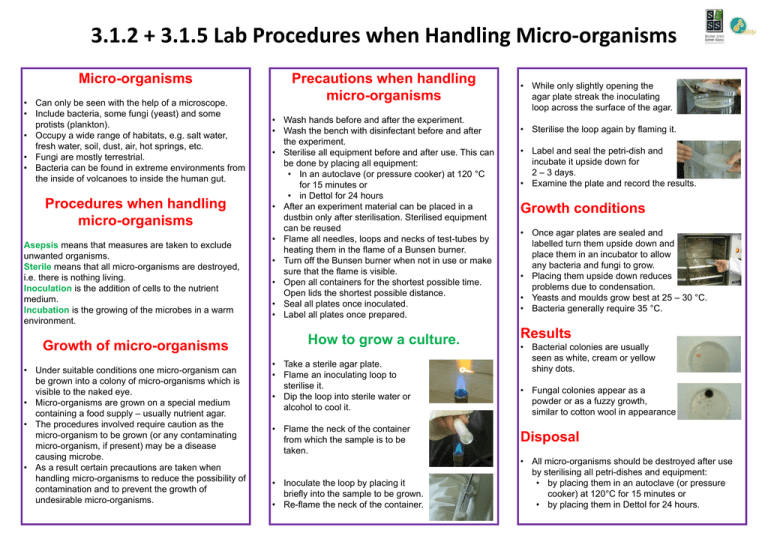
3.1.2 + 3.1.5 Lab Procedures when Handling Micro-organisms Micro-organisms • Can only be seen with the help of a microscope. • Include bacteria, some fungi (yeast) and some protists (plankton). • Occupy a wide range of habitats, e.g. salt water, fresh water, soil, dust, air, hot springs, etc. • Fungi are mostly terrestrial. • Bacteria can be found in extreme environments from the inside of volcanoes to inside the human gut. Procedures when handling micro-organisms Asepsis means that measures are taken to exclude unwanted organisms. Sterile means that all micro-organisms are destroyed, i.e. there is nothing living. Inoculation is the addition of cells to the nutrient medium. Incubation is the growing of the microbes in a warm environment. Growth of micro-organisms • Under suitable conditions one micro-organism can be grown into a colony of micro-organisms which is visible to the naked eye. • Micro-organisms are grown on a special medium containing a food supply – usually nutrient agar. • The procedures involved require caution as the micro-organism to be grown (or any contaminating micro-organism, if present) may be a disease causing microbe. • As a result certain precautions are taken when handling micro-organisms to reduce the possibility of contamination and to prevent the growth of undesirable micro-organisms. Precautions when handling micro-organisms • Wash hands before and after the experiment. • Wash the bench with disinfectant before and after the experiment. • Sterilise all equipment before and after use. This can be done by placing all equipment: • In an autoclave (or pressure cooker) at 120 °C for 15 minutes or • in Dettol for 24 hours • After an experiment material can be placed in a dustbin only after sterilisation. Sterilised equipment can be reused • Flame all needles, loops and necks of test-tubes by heating them in the flame of a Bunsen burner. • Turn off the Bunsen burner when not in use or make sure that the flame is visible. • Open all containers for the shortest possible time. Open lids the shortest possible distance. • Seal all plates once inoculated. • Label all plates once prepared. How to grow a culture. • Take a sterile agar plate. • Flame an inoculating loop to sterilise it. • Dip the loop into sterile water or alcohol to cool it. • Flame the neck of the container from which the sample is to be taken. • Inoculate the loop by placing it briefly into the sample to be grown. • Re-flame the neck of the container. • While only slightly opening the agar plate streak the inoculating loop across the surface of the agar. • Sterilise the loop again by flaming it. • Label and seal the petri-dish and incubate it upside down for 2 – 3 days. • Examine the plate and record the results. Growth conditions • Once agar plates are sealed and labelled turn them upside down and place them in an incubator to allow any bacteria and fungi to grow. • Placing them upside down reduces problems due to condensation. • Yeasts and moulds grow best at 25 – 30 °C. • Bacteria generally require 35 °C. Results • Bacterial colonies are usually seen as white, cream or yellow shiny dots. • Fungal colonies appear as a powder or as a fuzzy growth, similar to cotton wool in appearance Disposal • All micro-organisms should be destroyed after use by sterilising all petri-dishes and equipment: • by placing them in an autoclave (or pressure cooker) at 120°C for 15 minutes or • by placing them in Dettol for 24 hours.