Packet Unit 4 - Miss Clark's Website

Name : ___________________________________________________________________
Photosynthesis and Cellular Respiration Calendar
Unit 5; Chapter 8
11/1
11/4
11/5
11/6
Date
11/11
11/12
11/13 half day
11/14
11/15
11/18
11/19
11/20
Classwork
Photosynthesis survey
Notes: Photosynthesis PPT – Energy and Intro Photosynthesis
Color and Label the Chloroplast
Exit Slip
Photosynthesis and Elodea Demonstration (questions in class)
Go over confusion points (from yesterday)
Chlorophyll Graphing Activity
Demo Results, Day 2 (results and conclusion)
Go over chlorophyll graphing
Notes: Photosynthesis PPT – Details of Photosynthesis and
Review
Exit Slip
Photosynthesis with Elodea Lab
Go over confusion points (from yesterday)
Photosynthesis survey
Paper Chromatography Lab (go over)
Notes: Finish Photosynthesis (if needed) and start Cellular
Respiration
Finish Respiration PPT and Notes
Read through Respiration Lab
If time, start reading Lactic Acid
Begin Cellular Respiration in Yeast Lab
Pre-lab questions
Procedure writing and approval
Cellular Respiration in Yeast Lab
Conduct experiment
Review Day
Test
Packets Due
Homework (Due the next Day)
Read 8.1 and Answer Questions
Finish Chloroplast Coloring
Read 8.2 and Answer Questions
Complete Graph and questions
Answer Lab Questions
Finish Lab Questions
Read 8.3 and Answer Questions
Finish Lactic Acid Essay and summarize the article in bullet points
Finish Lab and study for the test
Check your lab for completeness
Study for test
Study for test
Rubric:
Photosynthesis Reading Questions
Color and Label the Chloroplast
Chlorophyll Graphing Activity
Out of Your Score
10
10
10
Lactic Acid Essay 20
Notes Photosynthesis and Cell Respiration 20
Photosynthesis with Elodea Graph and
Questions 20
Paper Chromatography Lab
Cellular Respiration in Yeast Lab
20
20
1
Chapter 8.1
Before you Read
1.
State the first law of thermodynamic.
Reading Questions
2.
Name the organism that makes its own food.
3.
Compare the energy usage in anabolic and catabolic pathways.
4.
Identify the step in the pathway where energy is captured?
5.
Identify Circle the high-energy bond that is broken when ATP is converted to ADP
Chapter 8.2
Before you Read
1.
Identify one way cells can use glucose
2.
Name Which organism has chloroplasts?
3.
Illustrate: Draw a picture of the thylakoid, grana and stroma
4.
Why do the leaves of some trees change color in autumn?
5.
What are photosystems I and photosystems II made of
6.
Highlight the path that electrons follow. What molecule is the electrons final destination?
7.
Name the main energy storing products of each phase of photosynthesis
8.
Name two places where CAM plants live
2
Chapter 8.3
Before you Read
1.
In the Krebs cycle, what is pyruvate converted to?
2.
Where does each of the following processes take place? a.
Glycolysis b.
Krebs Cycle c.
Electron Transport Chain
3.
What two processes make up anaerobic respiration?
4.
What types of organisms have cells that carry out all of the processes shown?
3
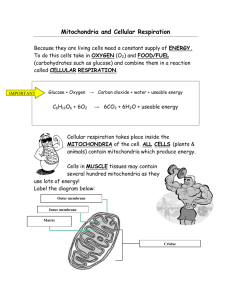
Color and Label the Chloroplast
4
PowerPoint Notes
Why do we eat?
Lunch (The Big Picture)
5
Energy Transformations
• Energy- is the ability to do ______________
(you love physics)
• Thermodynamics- the study of how energy
_________________ and
_________________ in the universe
Laws of Thermodynamics
• 1st Law of Thermodynamics- Energy can change form, but it can not be _________________ or destroyed
– When you eat- The _________________ energy in your food changes to _________________ energy
(movement)
• 2nd Law of Thermodynamics- Systems go from order to _________________ (entropy)
– When your body changes the energy from chemical to mechanical, some energy is
______________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
Energy can come from the Sun
• _________________ -get their energy by eating food
• _________________ -make their energy from the sun
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
6
Metabolism
• _________________ - all of the chemical reactions that take place inside cells
• Metabolic pathway- a series of reactions where the _________________ of one reaction is the _________________ for the next
Two types of Metabolic Pathways
• Catabolic- energy is _________________ by
_________________ _________________ molecules into small molecules
• Anabolic- energy is used to
_________________ _________________ molecules from small ones
• Energy from catabolic pathways fuel anabolic pathways
The link between plants and animals
• Photosynthesis— _________________ energy from the Sun is converted to
_________________ energy for use by the cell
• Cellular respiration— _________________ molecules are broken down to
_________________ energy for use by the cell
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
7
ATP: The Unit of Cellular Energy
• ATP
_________________ energy when the bond between the second and third phosphate groups is broken
Photosynthesis
• Two phases
– ____________________________________ –
Light energy absorbed and changed into chemical energy
– Light independent Reaction – chemical energy changes into a _________________ ___________
(glucose)
The Light Reaction
• First light is absorbed by _________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
8
Thylakoids
• Thylakoids contain light _________________ pigments
• Pigments absorb _________________ and reflect others
• We see the
_________________ color
Electron Transport
Dark Reaction- Calvin Cycle
• Calvin cycle - energy is _________________ in organic molecules such as glucose
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
9
Overview of Cellular Respiration
• Organisms obtain energy in a process called
_________________ _________________ .
• The equation for cellular respiration is the
_________________ of the equation for photosynthesis.
Two Parts
• _________________
• _________________ _________________
Glycolysis
• Glucose is broken down in the cytoplasm
• Takes place in ALL Cells
–
–
–
–
–
• Glucose converted into _____ pyruvate, 2 ATP and 2NADH are produced
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
10
Anaerobic- _______ OXYGEN
• Two main types
– Lactic acid fermentation
– Alcohol fermentation
• Anaerobic Respiration- takes place when there are no mitochondria (bacteria) or oxygen is not available
• Downside __________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
Aerobic Respiration
Krebs Cycle
• Most of the energy is still in the
_________________
• The net yield from the
Krebs cycle is
– 6 _________________
– 2 _________________
(only useable energy)
– 8 NADH
– 2 FADH
2
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
11
Electron Transport
• Final step in the breakdown of glucose
• ~______ ATP produced
• ______ is the final electron acceptor = _____
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
Overview of Cellular Respiration
Location Main Activity
High Energy
Molecules
Made
Glycolysis
Krebs
Cycle
ETC
12
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
___________________________________
13
Photosynthesis Demonstration
Purpose:
To show that plants use carbon dioxide (CO
2
) from their environments in the process of photosynthesis
Background:
Green plants use sunlight, carbon dioxide and water to make glucose (C
6
H
12
O
6
).
To release energy, the glucose must be converted into ATP by means of cellular respiration.
The waste products of cellular respiration are CO
2
and H
2
O.
CO2 dissolves in water to form a weak acid called Carbonic Acid.
An acid-base indicator (bromothymol blue) can be used to indicate the presence of CO2 in the water.
How it works
Exhaled air contains roughly 18% Oxygen, 78% Nitrogen and 4% Carbon Dioxide.
Bromothymol blue changes colors when in the presence of an acid or base.
Since exhaled air is partly composed of Carbon Dioxide, the CO2 combines with the water, forming
Carbonic Acid (H2CO3)
Carbonic Acid, being slightly acidic, turns the color of the Bromothymol blue solution yellow.
Photosynthesis
Photosynthesis uses sunlight, carbon dioxide and water to form glucose and oxygen.
Elodea is a water-dwelling plant that uses photosynthesis to remove carbon dioxide from water.
Since carbon dioxide makes water acidic, the process of removing the acid decreases the acidity of the water.
Once there is no more acid component to the solution, the bromothymol blue turns back to its original blue state.
Procedure
Experimental test tube: o Fill a test tube 2/3 of the way with water. Add bromothymol blue solution. o Using a straw, blow bubbles into the water. (the solution should turn yellow.) o Put a sprig of elodea in the test tube. o Cover with parafilm and let sit overnight.
Control test tube: o Fill a test tube 2/3 of the way with water. Add bromothymol blue solution. o Using a straw, blow bubbles into the water. (the solution should turn yellow.) o Put a sprig of elodea in the test tube. o Cover with parafilm. o Wrap entire test tube in aluminum foil and let sit overnight.
14
Questions:
Why should the bromothymol blue solution turn yellow after you blow bubbles into it?
Why did we wrap the control test tube in aluminum foil? (hint: what does the foil prevent from entering the test tube?)
Results:
What happened in the experimental test tube?
What happened in the control test tube?
Conclusion:
Explain your observations in terms of photosynthesis.
15
Chlorophyll Graphing Activity
1.
Color the graph above using the chart on the right.
2.
What color is least absorbed by chlorophyll a?
3.
What color is least absorbed by chlorophyll b?
4.
What color is most absorbed by chlorophyll a?
5.
What color is most absorbed by chlorophyll b?
6.
Beta carotene has its highest absorbance between 400nm and 550nm. It has zero absorbance under
400nm and over 550nm. a.
Graph the absorbance of beta carotene on the graph with chlorophyll a and chlorophyll b.
Make sure you label the graph. b.
What color(s) is plant tissue that is high in beta carotene? c.
What color(s) does beta carotene absorb?
7.
What foods are high in beta carotene (name 3)?
8.
Do you think that some plant pigments may absorb colors we cannot see? Explain.
16
Use bullet notes to summarize the main points of the following article. Space is provided for you on page 17.
You should include information about what scientists and trainers used to think, what they think now, how they came to their current conclusions, information about the key scientists in this article, and anything else that you find important.
Lactic Acid Is Not Muscles' Foe, It's Fuel
By GINA KOLATA
Published: May 16, 2006
Everyone who has even thought about exercising has heard the warnings about lactic acid. It builds up in your muscles. It is what makes your muscles burn. Its buildup is what makes your muscles tire and give out.
Coaches and personal trainers tell athletes and exercisers that they have to learn to work out at just below their "lactic threshold," that point of diminishing returns when lactic acid starts to accumulate. Some athletes even have blood tests to find their personal lactic thresholds.
But that, it turns out, is all wrong. Lactic acid is actually a fuel, not a caustic waste product. Muscles make it deliberately, producing it from glucose, and they burn it to obtain energy. The reason trained athletes can perform so hard and so long is because their intense training causes their muscles to adapt so they more readily and efficiently absorb lactic acid.
The notion that lactic acid was bad took hold more than a century ago, said George A. Brooks, a professor in the department of integrative biology at the University of California , Berkeley. It stuck because it seemed to make so much sense.
"It's one of the classic mistakes in the history of science," Dr. Brooks said.
Its origins lie in a study by a Nobel laureate, Otto Meyerhof, who in the early years of the 20th century cut a frog in half and put its bottom half in a jar. The frog's muscles had no circulation — no source of oxygen or energy.
Dr. Myerhoff gave the frog's leg electric shocks to make the muscles contract, but after a few twitches, the muscles stopped moving. Then, when Dr. Myerhoff examined the muscles, he discovered that they were bathed in lactic acid.
A theory was born. Lack of oxygen to muscles leads to lactic acid, leads to fatigue.
Athletes were told that they should spend most of their effort exercising aerobically, using glucose as a fuel. If they tried to spend too much time exercising harder, in the anaerobic zone, they were told, they would pay a price, that lactic acid would accumulate in the muscles, forcing them to stop.
Few scientists questioned this view, Dr. Brooks said. But, he said, he became interested in it in the 1960's, when he was running track at Queens College and his coach told him that his performance was limited by a buildup of lactic acid.
When he graduated and began working on a Ph.D. in exercise physiology, he decided to study the lactic acid hypothesis for his dissertation.
17
"I gave rats radioactive lactic acid, and I found that they burned it faster than anything else I could give them,"
Dr. Brooks said.
It looked as if lactic acid was there for a reason. It was a source of energy.
Dr. Brooks said he published the finding in the late 70's. Other researchers challenged him at meetings and in print.
"I had huge fights, I had terrible trouble getting my grants funded, I had my papers rejected," Dr. Brooks recalled. But he soldiered on, conducting more elaborate studies with rats and, years later, moving on to humans. Every time, with every study, his results were consistent with his radical idea.
Eventually, other researchers confirmed the work. And gradually, the thinking among exercise physiologists began to change.
"The evidence has continued to mount," said L. Bruce Gladden, a professor of health and human performance at
Auburn University. "It became clear that it is not so simple as to say, Lactic acid is a bad thing and it causes fatigue."
As for the idea that lactic acid causes muscle soreness, Dr. Gladden said, that never made sense.
"Lactic acid will be gone from your muscles within an hour of exercise," he said. "You get sore one to three days later. The time frame is not consistent, and the mechanisms have not been found."
The understanding now is that muscle cells convert glucose or glycogen to lactic acid. The lactic acid is taken up and used as a fuel by mitochondria, the energy factories in muscle cells.
Mitochondria even have a special transporter protein to move the substance into them, Dr. Brooks found.
Intense training makes a difference, he said, because it can make double the mitochondrial mass.
It is clear that the old lactic acid theory cannot explain what is happening to muscles, Dr. Brooks and others said.
Yet, Dr. Brooks said, even though coaches often believed in the myth of the lactic acid threshold, they ended up training athletes in the best way possible to increase their mitochondria. "Coaches have understood things the scientists didn't," he said.
Through trial and error, coaches learned that athletic performance improved when athletes worked on endurance, running longer and longer distances, for example.
That, it turns out, increased the mass of their muscle mitochondria, letting them burn more lactic acid and allowing the muscles to work harder and longer.
Just before a race, coaches often tell athletes to train very hard in brief spurts.
That extra stress increases the mitochondria mass even more, Dr. Brooks said, and is the reason for improved performance.
And the scientists?
They took much longer to figure it out.
"They said, 'You're anaerobic, you need more oxygen,' " Dr. Brooks said. "The scientists were stuck in 1920."
18
Article summary:
What they used to think:
Why did they think that?:
What they think now:
Why? What led them to this new conclusion?:
Key scientists’ names and roles:
Something that you thought was interesting:
19
Photosynthesis Diagram
20
Light Reaction Dark Reaction
21
Photosynthesis with Elodea Lab
Photosynthesis is the process by which plants take carbon dioxide from the atmosphere, add water, and use the energy of sunlight to produce sugar.
Write the equation for photosynthesis:
Photosynthesis occurs in the chloroplast, an organelle in plant cells that contains the molecule chlorophyll.
Chlorophyll absorbs the energy of sunlight. That light energy is converted to chemical energy through the steps of photosynthesis.
The reactions of photosynthesis can be divided into two major types: light-dependent reactions and lightindependent reactions. The light-dependent reactions convert energy from the sun into a form that the chloroplast can then use to make sugar from carbon dioxide, in the process producing oxygen as a waste product. The light-independent reactions use that energy to make glucose from carbon dioxide and water.
Materials:
Test Tube
Elodea cuttings
Sodium bicarbonate (baking soda)
Beaker with water
Lamp (found in the bottom cabinet of your lab station)
Procedure:
1.
Obtain a sprig of elodea. Remove several leaves from around the cut end of the stem. Slice off a portion of the stem at an angle and lightly crush the cut end of the stem.
2.
Place the sprig in a test tube, cut side up. Add water to test tube and a pinch of baking soda.
3.
Place the test tube into a beaker filled with tap water.
4.
Place a lamp next to the beaker. - The water in the beaker will help to absorb the heat from the light, thus reducing the variables in the experiment.
5.
Turn on the lamp. As soon as you see small bubbles coming from the cut end of the stem, time the reaction for 10 minutes. If you do not see bubbles, cut the stem again and recrush.
6.
Calculate the net photosynthesis in bubbles/min. (Divide the number of bubbles by 10 minutes.)
7.
Remove your test tube from the bright light. Observe and record the rate of bubbles without direct light.
22
Data:
Bright Light
Bubbles Total________/10
Bubbles/min __________
Dim Light
Bubbles Total_________/10
Bubbles/min ____________
Analysis: Complete in paragraph form
1.
Create a graph using your data
2.
What are the bubbles? Explain why bubbles happen.
3.
Did the number of bubbles change when the light intensity was reduced? Explain why this would occur?
4.
Why was the test tube placed in a beaker of water? What is a variable and why is it important to eliminate them?
5.
What was the purpose of adding sodium bicarbonate (baking soda) to the plant? Hint: look at the formula for photosynthesis
23
Paper Chromatography Lab
WEAR YOUR
Pigments in the chloroplasts absorb light energy during the process of photosynthesis. The light is converted to glucose, a sugar (chemical energy). Chlorophyll a and chlorophyll b absorb light in the blue and violet range. Other pigments such as carotenes and xanthophylls absorb light in the orange, red and yellow range.
Chromatography is a method used to separate different kinds of molecules from each other based on size and solubility in a given solvent. Smaller, more soluble molecules will move faster and further than large less soluble molecules.
In this lab, paper chromatography will be used to separate the photosynthetic pigments present in spinach leaves.
Procedure:
1.
Obtain a piece of chromatography paper. ONLY TOUCH THE PAPER BY THE EDGES! The oils in your fingers will damage the paper.
2.
Make a pointed end in the chromatography paper by snipping the edges off one end
3.
Measure 3cm from the pointed end and draw a light pencil line horizontally
4.
Lay the spinach leaf across the line
5.
Rub the penny across the spinach to in the direction of the line to cover the line with pigment from the spinach
6.
Add a small amount of chromatography solvent to a test tube a.
Caution when handling solvents this solvent is flammable
7.
Place the Chromatography paper into the test tube point down. The point should be
touching the solvent, but the pencil line should be above the solvent.
8.
Watch the solvent move up the paper, when the solvent is close to the top remove the paper from the test tube and allow it to air dry
9.
Tape the strip to a piece of paper or draw it on the paper to the left and label the four
bands of pigment a.
Yellow/Orange band is the carotenes b.
Yellow near the bottom is the xanthophylls c.
Below that is chlorophyll a and chlorophyll b. They are both green, but different shades.
24
Questions:
1. Sketch your results to the right:
2. Why is it beneficial to have pigments that absorb different wavelengths of light rather than only one color?
3. List the pigments in order from least to greatest solubility.
4. Do you think this would be true for all solvents? Hint You can try this by running the same experiment in
H2O
5. Design an experiment to test the colors in a water soluble black marker. Include a hypothesis, materials, procedure and expected results.
You can get 5 extra points for performing the experiment and attaching your results. You should have all of the materials at home or in class.
25
Cellular Respiration in Yeast Lab
Adapted from “Alcoholic Fermentation in Yeast Investigation” © 2009 by Dr. Jennifer Doherty and Dr. Ingrid Waldron, University of Pennsylvania Biology Department 1
All living cells, including the cells in your body and the cells in yeast, need energy for cellular processes such as pumping molecules into or out of the cell or synthesizing needed molecules. ATP is a special molecule which provides energy in a form that cells can use for cellular processes.
Cellular respiration is the process that cells use to transfer energy from the organic molecules in food to
ATP. The following equation summarizes the chemical changes that occur in cellular respiration of the monosaccharide glucose when oxygen is available.
C
6
H
12
O
6
+ 6 O
2
+ light 6 CO
2
+ 6 H
2
O + ATP glucose oxygen carbon wáter energy
gas dioxide gas
The chemical reactions in cellular respiration are similar to the chemical reactions when organic compounds are burned, but of course no ATP is produced. Instead energy is released in the form of light and heat. The following equation shows the chemical changes that occur when the monosaccharide glucose is burned.
glucose oxygen carbon water energy
gas dioxide gas
C
6
H
12
O
6
+ 6 O
2
6 CO
2
+ 6 H
2
O + heat
1.
What are the similarities between this equation for burning glucose and the equation for cellular respiration of glucose when oxygen is available?
2.
What is the difference between these equations?
26
Cellular respiration involves many small steps. These multiple steps allow the cell to use the energy from each glucose molecule efficiently in order to make as many ATP molecules as possible.
The first major step in cellular respiration is glycolysis 1 glucose 2 pyruvate + 2 ATP
What happens next depends on whether or not oxygen is available to the cells. When oxygen is available, cells can use the Krebs cycle and the electron transport chain to make up to 36 ATPs
2 pyruvate + 6 O
2
6 CO
2
+ 36 ATP
Cellular respiration that uses O
2
is called aerobic respiration. Most of the time, the cells in our bodies use aerobic respiration:
When oxygen is not available, cells use anaerobic processes to produce ATP. (The "an" in front of aerobic means “not”, so anaerobic means "not aerobic".)
Under anaerobic conditions, many cells use a process called fermentation to make ATP. There are 2 types of fermentation
lactate fermentation (e.g. in muscles when an animal exercises hard) and
alcoholic fermentation (e.g. by yeast to make wine and beer).
Fermentation has two disadvantages compared to aerobic respiration. Fermentation produces less ATP than aerobic respiration, and fermentation produces a toxic byproduct (either lactate, which becomes lactic acid, or alcohol). However, fermentation is very useful if oxygen is not available.
27
3.
Use the previous information to complete the figures below. Fill in the ovals with the appropriate molecule. On the blank lines write the name of the appropriate process. In the boxes at the bottom of the figure write how much ATP is made in each pathway.
Humans use yeast every day. What is yeast? What are some common uses of yeast?
If you want to make your own bread, you can buy yeast in the grocery store. This yeast consists of little brown grains. The little brown grains of yeast may not seem to be alive, but if you put them in water with sugar, the yeast will carry out cellular respiration and grow.
4.
You can grow yeast in a test tube filled with water and sealed with a balloon. Do you think these growth conditions are aerobic or anaerobic?
5.
Under anaerobic conditions, yeast carries out alcoholic fermentation, so it produces
_________________ and ____________________. You can measure the rate of fermentation in yeast by measuring the amount of carbon dioxide gas the yeast produces. Carbon dioxide production can be measured by measuring the depth of the layer of bubbles trapped in foam on top of the yeast solution and also by observing the balloons, which catch the carbon dioxide produced and get bigger.
28
Part I - Sucrose Concentration
6.
What is sucrose?
Your first experiment will investigate the effect of sucrose concentration on the rate of cellular respiration in yeast. Yeast can convert sucrose into glucose and use it during cellular respiration.
You will design an experiment to answer the question: Does the concentration of sucrose affect the rate of cellular respiration in yeast?
Your teacher will provide you with yeast, test tubes, balloons, rulers, and four concentrations of sucrose water: 0% (plain water), 1%, 5% and 10% sucrose.
7.
Write a hypothesis that you will test to help you answer the research question.
8.
What will be the independent variable in your experiment?
9.
What will be the dependent variable in your experiment?
10.
What will be the control treatment in your experiment?
What is the purpose of this control treatment?
29
11.
The basic procedure to measure cellular respiration is:
1) Add 25 mL of the appropriate sucrose solution to each tube.
2) Add ¼ tsp of yeast to each tube.
3) Put a balloon on the top of each tube.
4) With your palm sealing the top, shake each tube until the yeast is dissolved.
5) Measure the depth of bubbles produced and observe how the balloons change after 10 minutes and 20 minutes.
Write your specific procedures here:
12.
Complete the first column of these data tables. Record your data below:
Depth of CO
2
bubbles in:
Sucrose treatment
10 minutes 20 minutes
Balloon description
Sucrose treatment
10 minutes 20 minutes
30
13.
Did the yeast produce different amounts of carbon dioxide with different sucrose concentrations?
Do the results match your hypothesis?
14.
Discuss your results with your group. What conclusions concerning the relationship between sucrose concentration and the rate of cellular respiration are supported by your results?
31
Extra Credit Yeast and Other Ingredients in Bread
Due ____________ Grade 40/40 on a packet
Do this with one family member, and explain each step to them. Write answer to bold questions on a
separate sheet of paper
and turn in a picture of you and your family member with a photo.
Family member name____________________________
When you make bread, if you just mix flour, sugar and water, the dough does not rise, and the bread will be flat and hard. If you include yeast in the bread dough, then the dough rises and the bread is bigger and fluffier.
1.
Explain how the yeast helps the bread dough to rise?
Consider the results of your last experiment with yeast and sucrose.
2.
If you added flour, which treatment would have made the fluffiest bread?
Today you will design and carry out an experiment to investigate other variables, besides the concentration of sugar, which may affect the fluffiness of bread. Bread dough usually has other ingredients besides yeast, sugar, water and flour. Some other common ingredients in bread dough are salt, fats (e.g. oil or butter), eggs, and flavorings such as cinnamon and raisins. Any of these ingredients could affect the rate of fermentation of the yeast and thus affect the fluffiness of the bread. The temperature at which the bread dough is kept to rise might also affect the fluffiness of bread.
You will not actually test how one of these ingredients or temperature affects the fluffiness of bread.
Instead, you will use the same experimental setup as before (that is, test tubes with yeast mixture, a ruler and balloons) to test the effect of one of these variables on the rate of CO
2
production.
3.
What question will you investigate?
4.
Write a hypothesis that you will test to help you answer this question.
5.
Plan an experiment to test your hypothesis.
6.
What is the independent variable in your experiment?
7.
What is the dependent variable in your experiment?
8.
What is the control treatment in your experiment? (Hint: You are not testing how sugar affects cellular respiration, so you will want to use 10% sucrose in each of your treatments)
9.
What are your procedures?
10.
Create a data table.
11.
Perform your experiment and collect your data.
12.
Did the yeast produce different amounts of carbon dioxide with different treatments? Do the results match your hypothesis?
13.
What do your results mean for people who make bread using the ingredient or temperature you investigated?
You can bake your bread in the oven at 350 o F until golden brown. Enjoy
32