Solutions Review
advertisement

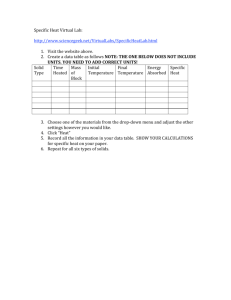
Thermochemistry 4 Boon Chemistry February 4 & 5, 2013 Catalyst Take out your homework please. According to the heat curve for Objectives • I can calculate the amount of water shown below: energy transferred as heat during a Does the system absorb or release phase change. heat as ice melts? Does the temperature of water Agenda increase when it is melting? Catalyst What phase change happens at #4? Exit Slip Review Notes: Latent Heat of Phase Change Practice Calculations and heat curve analysis Exit Slip: Calculations Phase Diagram Review #5: When #2: The temperature water freezes remains the kinetic constant energy whenofthe water is a mixture molecules decreases. of ice Theand molecules water because move the energy isThis slower. being is exothermic used to meltbecause the ice.energy is released. (1) Ice; temperature is increasing (2) Ice and water; temperature is constant (3) water; temperature is increasing (4) Water & steam; temperature is constant #4: When water boils the kinetic energy of molecules increases. The molecules move faster. This is endothermic because it requires energy. (5) steam; temperature is increasing Exit Slip Review 7. Which of the following statements best describes the relationship between the transfer of heat and temperature? (a) The transfer of heat always raises temperature. (b) The transfer of heat never causes a change in temperature. (c) The transfer of heat always decreases temperature. (d) The transfer of heat does not always cause a change in temperature. 8. What happens to the temperature of matter as energy is added during a change of state? (a) temperature increases (b) temperature decreases (c) temperature does not change (d) temperature changes depending on the material 9. List the names two phase changes that result in a release of energy to the surroundings: Freezing, condensation, deposition 10. List the names of two phase changes that result in an absorption of energy from the surroundings: Vaporization, fusion, sublimation Exit Slip Review Exothermic 2NaHCO3 + energy→ Na2Co3 + H2O + CO2 Endothermic Exit Slip Review 3. In an endothermic process, energy is (absorbed/released). 4. In an exothermic process, energy is (absorbed/released). 5. Which of the following is an example of energy: (a) color (c) copper (b) heat (d) temperature Why? Heat is one way that energy is transferred. Temperature is a measure of the average kinetic energy of particles in an object. But it is not energy itself. 6. When energy is added to liquid water, what happens? (a) The molecules move and vibrate less. (b) There is no change in the motion of the molecules. (c) The molecules move and vibrate more. (d) The water becomes ice. Specific Heat Review: Use your notes to do the following specific heat problem. 3000 g of water are heated from 50 °C to 99°C. The specific heat of water is 4.18 J/g*K. How much heat was absorbed? Notes: Heat Transfer During Phase Changes We discussed the fact that temperature does not increase during a phase change. Instead, all the energy that is released or absorbed is used to power the phase change. On the phase change diagram this portion of the heating curve has a slope of zero. Today we will calculate how much heat is absorbed or released during a phase change. We will use water as our example, but a similar calculation can be done for other substances. What do you think? Which requires more energy to melt? The amount of heat absorbed or released during a phase change is generally called the latent heat of transformation. Different substances have different latent heats of transformation. Also, different phase changes absorb or release different amounts of heat. For example, less energy is absorbed to melt ice than is absorbed when water is vaporized. Table 1 contains the heats of transformation for water. Analyzing Data. How do the heats of transformation for water compare to the specific heat of water? How many joules are released when 1 gram of water freezes? Which requires more energy: melting or vaporization? Why is the heat of fusion the same as the heat of crystallization? Latent Heat of Transformation Calculations If we know the latent heat of transformation of a substance, we can calculate the amount of heat energy absorbed or released during a phase change. The formula: Q = m × ΔHtranformation Phase Change Calculations Example 1: How much heat is required to melt 1000 g of ice at 0°C? Phase Change Calculations Example 2: How much heat is needed to vaporize 200 g of water at 100°C? Heat Transfer Calculations Example 3: How much ice, at 0°C, can be melted by 100 J of heat? Work Time 1. Complete today’s notes worksheet 2. Get a stamp when you are done and check your answers. 3. Work on the phase change diagram worksheet (passed out today). This is due Friday. 4. Work on any worksheets that you have not completed from last week. These are due Friday. Exit Slip Expectations: Tools: You may use all your notes, worksheets, and flash cards. You may use your own calculator. What do I turn in? You will work silently and independently. When you are done, cover your exit slip with your handouts. You will turn in your exit slip only. Homework: Complete any worksheets or article questions that you have not finished. Homework Due Thurs/Fri (Feb. 7 & 8): All Thermochemistry notes and handouts.