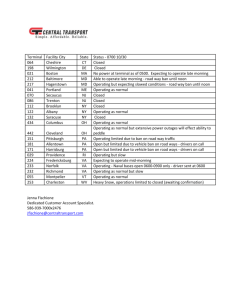
Module 4-9 - Workforce Solutions
advertisement

Module 4 1 History of Good Clinical Practice Regulations Barbara Novak 2 US Food and Drug Law Chronology • • 1848 Congress banned importation of adulterated drugs after a bad batch of quinine was imported for U.S. Army. (Did not affect domestic issues of adulteration) 1879 Congress began investigating incidents involving contamination of food and drugs through the USDA BAN/CDR 3 US Food and Drug Law Chronology • 1902 Biologics Control Act – Required licensing of serums and vaccines • 1906 Pure Food and Drug Act – Congress began investigating incidents involving contamination of food and drugs through the USDA BAN/CDR 4 1906 Pure Food and Drugs Act AKA Wiley or Heyburn Act • Dr. Wiley’s lunch crew • Upton Sinclair’s The Jungle • Prohibited interstate transportation of adulterated and misbranded foods and drugs BAN/CDR 5 US Food and Drug Law Chronology • 1912 Sherley Amendment – Prohibited false and fraudulent curative claims or therapeutic claims on labels (snake venom, elixirs, wonder cures) • 1938 Food, Drug and Cosmetic Act – Added cosmetics to the 1906 Act BAN/CDR 6 1938 Food, Drug and Cosmetic Act • Elixir of Sulfanilamide - 1937 • Required pre-market approval of new drugs for safety BAN/CDR 7 Food, Drug and Cosmetic Act • Authorizes FDA to inspect at reasonable times • Authorizes FDA to promulgate and enforce regulations BAN/CDR 8 US Food and Drug Law Chronology • 1962 Kefauver Act – Amendments to the 1938 ACT that set up a mandatory reporting system for drug safety – Led to the Spontaneous Reporting System which catalogues ADR reports for marketed drugs BAN/CDR 9 1962 Kafauver Act • Thalidomide tragedy BAN/CDR 10 1987 IND Rewrite • 21 CFR Part 312 Introduced – Laid out the responsibilities of sponsors, monitors and investigators BAN/CDR 11 Prescription Drug User Fee Act (PDUFA) •1992 & 1997 •FY2001 Fees –Application fee - $309,647 –Establishment Fee - $145,987 –Annual product fee - $21,892 • FY 2005 Fees –Application fee - $672,000/ 336,00 (SNDA) –Establishment Fee - $262,000 –Annual product fee - $41,710 BAN/CDR 12 Good Clinical Practices (GCPs) • Phrase coined by industry • Includes Regulations, Guidelines and Industry practices which address responsibilities of: – the Sponsor – the Investigator – Institutional Review Boards and requirement for Informed Consent. BAN/CDR 13 Regulations vs Guidelines • Regulations = LAW • Guideline = Clarification BAN/CDR 14 Purpose of GCP’s • To protect the rights and safety of subjects • To assure the quality and integrity of the clinical research data BAN/CDR 15 GCP Regulations • 21 CFR Part 312 – IND Regulations • 21 CFR Part 312.50 to 312.59 – Sponsor/monitor responsibilities • 21 CFR Part 312.60 to 312.69 – Investigator responsibilities • 21 CFR Part 50 – Informed consent regulations BAN/CDR 16 GCP Regulations • 21 CFR Part 56 - IRB regulations • 21 CFR Part 11 – Electronic signatures • 21 CFR Part 54 – Financial disclosure BAN/CDR 17 GCP Guidelines • FDA Information Sheets • Guidelines for Monitoring, Investigators, etc • ICH GCP Guideline BAN/CDR 18 FDA Organization • FDA is 1 agency within the Dept. of Health and Human Services (HHS) – Reports to Secretary of HHS – Funding is through the HHS budget (FDA has a separate budget) – Small agency – frequent visit to Capitol Hill BAN/CDR 19 FDA • FDA is comprised of 8 Centers/Offices CDER – Center for Drug Evaluation and Research (most therapeutic biologics recently transferred to CDER) CBER- Center for Biologics Evaluation and Research) CDRH – Center for Devices and Radiological Health BAN/CDR 20 FDA CFSAN – Center for Food Safety and Applied Nutrition CVM – Center for Veterinary Medicine NCTR – Nat’l Center for Toxicological Research OC - Office of Commissioner (head of FDA) ORA – Office of Regulatory Affairs BAN/CDR 21 Office of Medical Policy • Reports to CDER Director • Division of Scientific Investigations – Select sites for inspection – Assist on inspections – Review inspection reports – Final classification of reports/regulatory action BAN/CDR 22 Office of Regulatory Affairs (ORA) • Associate Commissioner for Regulatory Affairs • Office of Resource Management • Office of Regional Operations • Office of Enforcement • Office of Criminal Investigations BAN/CDR 23 Field Set-Up • Report to Office of Regional Operations • 5 Regions – Northeast Region • Regional office in New England & NY • District offices in NE, NYC, Buffalo – Central Region • Regional office in Phila, Baltimore & Chicago • District offices in Philadelphia, Baltimore, Chicago, NJ, Cincinnati, Detroit, Minneapolis BAN/CDR 24 Field Set-Up – Southeast Region • Regional office in Atlanta • District offices in Atlanta, New Orleans, Florida, San Juan – Southwest Region • Regional office in Dallas, Denver, KC • District offices in Dallas, Denver, Kansas City, and a Southwest Import District BAN/CDR 25 Field Set-Up – Pacific Region • Regional office in San Francisco & Seattle • District offices in Los Angeles, San Francisco & Seattle BAN/CDR 26 Submissions • Drugs – IND: Investigational New Drug Application – NDA: New Drug Application • Biologics – BB-IND: Biologics Investigational Drug Application – BLA: Biologics License Application BAN/CDR 27 FDA Website • www.fda.gov • Each center is represented • Can access guidelines, regulations, speeches • Warning letters are posted, and investigator disqualifications BAN/CDR 28 Module 9 29 International Conference on Harmonization (ICH) • US, EU, Japan • Industry, trade organizations and regulatory representatives • 5 step process, with Step 5 being FINALIZATION of the individual guideline – i.e., acceptance of the guideline by each country BAN/CDR 30 Purpose of ICH • • • • • • Harmonize standards Improve quality and reliability of data Protect rights & welfare of subjects Minimize human new drug exposure Speed up marketing of new drugs Decrease cost BAN/CDR 31 ICH GCP Guideline • Published as guideline in US • Includes requirements for – Sponsors – Investigators – IRB/IEC – Informed consent – Essential documents BAN/CDR 32 ICH GCP Guideline • Similar to FDA regulations • FDA very involved in ICH process • More detailed in most places BAN/CDR 33 FDA Bioresearch Monitoring Program • Kefauver Harris amendments • Field survey 1972 – 1974 • Sponsors, Investigators, Contract research Organizations (CROs), IRBs, Toxicology Laboratories • Drugs, Devices, Biologics, Foods, Veterinary Medicine BAN/CDR 34 Why GCPs? • • • • Mandated by regulatory authorities History indicates need Uniform way of conducting research Drug/biologic/device development is expensive – DO IT RIGHT THE FIRST TIME BAN/CDR 35 Office for Good Clinical Practice (OGCP) • • • • Within the Office of the Commissioner Agency-side coordinating functions Work with OHRP Improve conduct and oversight of clinical research • Ensure protection of participants in FDAregulated clinical research BAN/CDR 36 Responsibilities • Promote protection of human research participants • Support quality and integrity of clinical trials and applications • Support global harmonization and implementation of GCP standards BAN/CDR 37 HHS • OHRP – Office of Human Research Protections • More inspections of IRBs • Required training for sites • Loss of funding BAN/CDR 38 Module 8 39 Obligations of Sponsors 21CFR 312.50 to 312.59 DEFINITION Sponsor: An individual, company, institution or organization that takes responsibility for and initiates (and/or finances per ICH) a clinical investigation BAN/CDR 40 Obligations of Sponsors 21CFR 312.50 to 312.59 KEY POINTS: Select and Update Investigators Study Monitoring Recordkeeping Drug Distribution Maintain the Regulatory Submissions BAN/CDR 41 Obligations Of Sponsors 21CFR 312.50 to 312.59 SPECIFIC OBLIGATIONS: Sponsor commits to: Obtain a signed FDA form 1572 and Curriculum Vitae from each investigator Ensure investigator understands his/her obligations per the regulations Provide investigator with a current Investigator Brochure BAN/CDR 42 Obligations Of Sponsors 21CFR 312.50 to 312.59 SPECIFIC OBLIGATIONS: Sponsor commits to: Obtain sufficient, accurate financial information to submit the required certification or disclosure statements, and obtain commitment from investigator to update this information if relevant changes occur BAN/CDR 43 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Keep investigator informed of new observations discovered or reported, particularly with respect to adverse effects and safety Monitor study by on-site visits BAN/CDR 44 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Ensure that investigator and staff are following approved protocol and ensure that case report forms are being adequately completed Assure that qualified IRB will be involved Assure investigator will inform IRB of any protocol changes except to eliminate an immediate hazard to subjects BAN/CDR 45 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Evaluate evidence relating to safety and efficacy as it is received from the investigator Discontinue study if it is determined that the study drug presents an unreasonable and significant risk to subjects. Notify FDA, all IRBs and all investigators of a discontinuation for such reasons BAN/CDR 46 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Submit to FDA annual progress reports, updated Investigator Brochure, and IND safety reports as required Maintain records showing any financial payments to clinical investigators Maintain records of drug shipment, receipt, and disposition, including date, quantity, and batch BAN/CDR 47 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Assure the return or disposition of unused supplies Retain all records for 2 years after NDA is approved or study is discontinued Allow for FDA inspections of records and reports of clinical investigations BAN/CDR 48 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Discontinue drug shipment or secure compliance from any investigator who has not maintained or made available his/her records to FDA for inspection Assure compliance with storage and record-keeping requirements BAN/CDR 49 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Notify FDA and all participating investigators of any serious and unexpected adverse experience associated with the use of the study drug BAN/CDR 50 Obligations Of Sponsors 21CFR 312.50 to 312.59 Continued... Notify FDA and all participating investigators of any preclinical findings that suggest a significant risk for human subjects (e.g., mutagenicity, teratogenicity, or carcinogenicity findings) BAN/CDR 51 Obligations Of Clinical Investigators DEFINITION Investigator: An individual who actually conducts a clinical investigation (i.e., under whose immediate direction the drug is administered or dispense to a subject). If conducted by a team, the inv. is the responsible leader of the team. ICH: A person responsible for the conduct of the clinical trial at a site BAN/CDR 52 Obligations Of Clinical Investigators REGULATION: 21CFR 312.60 to 312.70 KEY POINTS: Conduct Clinical Study Protection of Patients Control of Drug Compliance with IRB requirements Recordkeeping Reporting BAN/CDR 53 Obligations Of Clinical Investigators Specific Obligations: The Investigator commits to: Personally conduct or supervise the described investigation Provide a list of the names of subinvestigators who will be assisting in the conduct of the investigation BAN/CDR 54 Obligations Of Clinical Investigators Continued… Ensure that all associates, colleagues, and employees assisting in the conduct of the study are informed about their study obligations. Such associates, colleagues, and employees must be listed on the FDA form 1572 BAN/CDR 55 Obligations Of Clinical Investigators Continued… Assure that study is conducted in accordance with the protocol, and only make changes after notifying the sponsor Obtain informed consent from all patients prior to their participation in the study BAN/CDR 56 Obligations Of Clinical Investigators Continued… Prepare and maintain adequate and accurate case histories that record all observations relevant to the study. Case histories include study CRFs, informed consent forms, and medical records including progress notes, hospital charts, nurses notes, and lab records BAN/CDR 57 Obligations Of Clinical Investigators Continued… Promptly report to the sponsor all adverse effects that may reasonably be suspected to have been caused by the study drug. If the adverse effect is serious and unexpected, it should be reported to the sponsor immediately BAN/CDR 58 Obligations Of Clinical Investigators Continued… Assure that study drug is administered only to subjects under the investigator’s personal supervision or the supervision of a subinvestigator in his/her study Return or otherwise dispose of all unused study medication in accordance with the sponsor’s instructions BAN/CDR 59 Obligations Of Clinical Investigators Continued… Maintain accurate and adequate records of drug disposition and drug accountability Assure that an IRB that complies with the IRB regulations will be responsible for the initial and continuing review and approval of the proposed protocol BAN/CDR 60 Obligations Of Clinical Investigators Continued… Assure that the study informed consent form is approved by an IRB prior to initiation of the study Assure that all changes in the protocol are reported to an IRB, and assure that no changes will be made to the protocol without prior IRB approval, except where necessary to eliminate apparent, immediate hazards to human subjects BAN/CDR 61 Obligations Of Clinical Investigators Continued… Submit annual progress reports to an IRB Submit progress reports of study findings to the sponsor Provide sponsor with a final study report shortly after completion of participation in the clinical investigation BAN/CDR 62 Obligations Of Clinical Investigators Continued… Maintain study records for 2 years after NDA approval or 2 years after discontinuation of study Provide sponsor with sufficient, accurate financial information to submit the required certification or disclosure statements, and obtain commitment from investigator to update this information if relevant changes occur BAN/CDR 63 Obligations Of Clinical Investigators Continued… Permit access to, including copying of study records and reports by FDA, sponsor, or sponsor-designated individual(s) BAN/CDR 64 Obligations Of Clinical Investigators Laboratory Records Form 1572 lists laboratory and its location Lab accreditation stored with investigator Lab normals retained Label lab samples with patient ID, study number and date BAN/CDR 65 Module 6 66 Obligations of IRBs DEFINITION: Institutional Review Board (IRB): any board, committee or group formally designated by an institution to review, approve and conduct periodic review of biomedical research involving human subjects. ICH definition: An independent body constituted of medical, scientific and nonscientific members whose responsibility it is to ensure the protection of the rights, safety and well-being of human subjects involved in a trial. BAN/CDR 67 Obligations of IRBs REGULATION: 21CFR 56 Purpose: Protect the rights and welfare of research subjects both before and during their study participation Determine: Should the study be approved (do benefits outweigh the risks)? What constitutes adequate informed consent? BAN/CDR 68 IRB Membership & Organization • At least five members with appropriate qualifications to review the research • At least one member whose primary area of interest is in a nonscientific area • At least one member who is independent of the institution/trial site • Should not consist entirely of men or women • A list of IRB members should be maintained BAN/CDR 69 IRB Functions • Reviews protocols, consents, and ads • Reviews amendments • Provides expedited review—i.e. minor changes to a protocol that don’t increase risk • Provides continuing review of trials (at least annually) • Reviews qualifications of investigators • Has the authority to suspend research • Reviews all adverse drug reactions both serious and unexpected BAN/CDR 70 IRB Records • Copies of research proposals/protocol (and accompanying documentation) • Minutes of meetings • Correspondence • List of members • Written operating procedures (SOPs) for IRB • Kept for 3 years after completion of research BAN/CDR 71 IRB: Expedited Review An IRB may use an expedited review procedure to review minor changes in previously approved research during the period (1 year) for which the approval is authorized Expedited review may be carried out by the IRB Chairperson or by one or more experienced reviewers designated by the IRB Chairperson from among the members of the IRB BAN/CDR 72 IRB: Expedited Review Procedures IRB’s which use expedited review must have a method of keeping all IRB members informed of research proposals which have been approved under this procedure FDA may restrict, suspend, or terminate an IRB’s use of expedited review when necessary to protect the rights of subjects BAN/CDR 73 Module 5 74 Informed Consents (IC) IC overview IC audit checklist Class examples of IC showing required elements FDA Warning Letters from IC Handout example of industry IC BAN/CDR 75 Informed Consent REGULATION: 21CFR 50 Purpose: Patient protection Patient information BAN/CDR 76 Informed Consent Process • Exchange of meaningful information • Begins with subject first learns of the study • Ends when there is no more new relevant information • Prior to the performance of study procedures BAN/CDR 77 Informed Consent Content Required Elements Additional Elements, as applicable Institution, local, state requirements Language understandable to subject Provide information subject needs to make an informed decision about participation BAN/CDR 78 Informed Consent Required Elements of Informed Consent: 21CFR 50.25 Purpose, duration and procedures of the research Foreseeable Risks to subject and benefits to subject or others Alternative procedures or courses of treatment Confidentiality of records – must note the possibility of FDA inspection or others BAN/CDR 79 Informed Consent Required Elements of Informed Consent: Explanation of compensation, if any, for research-related injury Contacts for pertinent questions or reports of research related injuries Statement of voluntary participation -No penalty or loss of benefit upon refusal to participate or withdrawal of consent BAN/CDR 80 Informed Consent Additional Elements of Informed Consent where applicable (required by ICH): May involve unforeseeable risks to subject (embryo/fetus) Circumstances in which the study may be terminated without subject’s consent Any additional costs to subject from participation BAN/CDR 81 Informed Consent Additional Elements of Informed Consent where applicable (required by ICH ): Consequences of early withdrawal Significant new findings during study Approximate number of subjects in study BAN/CDR 82 Informed Consent Problems 50% of FDA inspections at clinical investigator sites identify inadequacies in the informed consent Lack of required elements Lack of proper documentation Not obtained prior to enrollment Improper IRB approval BAN/CDR 83 HIPAA Health Insurance Portability and Accountability Act (HIPAA) -Enacted in 1996 to protect the security and confidentiality of electronic health information. -Primarily directed at the health care industry, insurers and medical providers. -Original deadline for privacy rule by Congress missed in 1999- finally published in Dec. 2000 , with a compliance date of April 14, 2003. BAN/CDR 84 HIPAA Law applies to “Covered Entities and Business Associates” that use or disclose protected health information. Requires the “de-identification” of individually identifiable health information, and disclosure of uses of protected health information (PHI). Although no FDA regulation specifically addresses HIPAA, clinical research certainly deals with PHI. BAN/CDR 85 HIPAA Clinical research now includes HIPAA authorization –either in the IC directly or in a separate document, depending on institutional or IRB policy. - must specifically give permission to use PHI for the purpose of research - have the right to withdraw permission to use PHI - expiration date of the authorization - suspension of access rights to study records until study is completed. BAN/CDR 86 Issues Noted in Recent Warning Letters and FDA 483’s Informed Consent Study procedures performed prior to signing and dating the IC Failure to obtain appropriate IC (e.g., no signature, incorrect signature/date, not in a language understandable to the subject) Not current/correct version of IC Elements missing from the IC BAN/CDR 87 Disclaimer • “This workforce solution was funded by a grant awarded under • the Workforce Innovation in Regional Development (WIRED) as implemented by the U.S. Department of Labor’s Employment and Training Administration. The solution was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership. This solution is copyrighted by the institution that created it. Internal use by an organization and/or personal use by an individual for non-commercial purposes is permissible. All other uses require the prior authorization of the copyright owner.” 88