PPT

Population-based
Interventions to
Improve Sexual
Health: Development and Evaluation
Colleen A. Redding, Ph.D.
Cancer Prevention Research Center
University of Rhode Island
How many people get new
STI’s in the U.S. every year?
Disease
All STI’s
HPV (Genital warts)
Trichomoniasis
Chlamydia
HSV (Genital herpes)
Gonorrhea
Syphilis
HIV
New cases/year
18.9 million
5.5 million
5 million
3 million
1 million
650,000
70,000
>40,000
Public Health Cost?
$9.3
–15.5 Billion per year in direct medical costs only
8 STIs
(HIV, HPV, HSV2, HepB, Chlamydia,
Gonorrhea, Syphillis, Trichomoniasis)
estimate is Y2000 $
$6.5 Billion among 15-24 yr. olds
(Chesson et al., 2004)
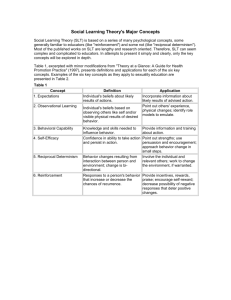
The Transtheoretical Model
Intentional Behavior Change
•
Stages of Change
•
Decisional Balance
•
Situational Efficacy / Temptations
•
Processes of Change
Different variables important for each stage transition
What are Expert
Systems?
A computer software program that codifies the reasoning of human experts into decision rules or algorithms
Integrates assessment and feedback consistently using decision rules
Different Levels of Targeting/Tailoring
Group Level Same intervention for all
Stage Level Targeted interventions
Precontemplation Contemplation Preparation Action Maintenance
Intermediate Level Tailored interventions
Individual LevelExpert system interventions
Stage-targeted vs. TTM-Tailored univariate group feedback clinical decisions
5 different types multivariate (10-15) individual feedback empirical decisions normative comparison ipsative comparison thousands of types interactive algorithms vary by Stg
Benefits of Expert System
Interventions
Provide highly individualized feedback
Appropriate for those at all stages of change,
(not only prepared to change)
Potentially cost-effective
Integrate multiple risk behaviors
Multimedia components
Confidentiality
Force explicit (testable) decision rules
High Fidelity
Efficacy of TTM-Tailored Interventions for Single Health Behaviors
Smoking Cessation
Healthy Diet
Physical Activity
Sun Protection
Medication Adherence
Stress/Depression Management
Mammography screening
School Bullying
Efficacy of TTM-Tailored Tx with
Multiple Behaviors
Smoking, Diet, Sun Protection
Smoking, Diet, Sun, Mammography
Smoking, Diet, Blood Glucose Monitoring
Smoking, Diet, Physical Activity, Stress
Diet, Physical Activity
Steps in the Intervention
Development Process
Focus Groups
Learn language and how participants think about the area.
Pilot Sample
Validate measurement structure of constructs
Normative database
Assess variables that differentiate stages
Develop Prototype – test - retest
Pilot test intervention
Efficacy/effectiveness trial (s)
CA Redding 1 , JO Prochaska 1 , JS Rossi 1 , K
Armstrong 2 , D Coviello 2 , UE Pallonen 1 , K
Evers 1 , WF Velicer 1 , & L Ruggiero 1
1 - Cancer Prevention Research Center, University of RI
2 - Family Planning Council, Philadelphia, PA
Human Papillomavirus - HPV
The most prevalent STI in the U.S.
Prevalence highest among 18-24 year old women (14% - 50%) (men not studied well)
Some HPV subtypes cause genital cancers
> 99% of cervical cancers have HPV DNA detected within the tumor
HPV associated with penile, anal, and oral cancers
New HPV vaccine protects against 4 types
Step By Step: Steppin’ for
Healthier Teens
4 urban family planning clinics –
Philadelphia metropolitan area
About 75% participation rate among eligible adolescents
833 female nonpregnant 14-17 y.o.
Teens - informed assent/consent parental consent not needed
Randomized clinical trial
Sample Diversity
(N=831)
Race/Ethnicity
Black / African-American
White / European-American
Hispanic / Latina
Native American
Other / Multiracial
%
81.0
7.3
7.8
1.4
1.8
Sexual Risks
Age of sexual debut 13-14 y.o.
Hx. Chlamydia
Hx. Gonorrhea
Hx. HPV, Herpes, or Syphilis
Hx. Pregnancy (at least one)
%
62.7
20.5
10.3
9.4
36.0
Urban Female Teens
(N=828)
Stages of Condom Adoption
35
30
25
20
15
10
5
0
PC C PR A M
13.6%
N=113
31.0%
N=257
15.0%
N=124
17.3%
N=143
23.1%
N=191
Pros and Cons of Condom Use
Weight of the positive and negative aspects of behavior change
PROS
BENEFITS
CONS
COSTS
REASONS
To use condoms NOT to use condoms
56
54
52
50
48
46
44
Functional Relationship
Stages & Pros + Cons
PC C PR A M
Pros
Cons
Baseline Sample - Pros & Cons of Condom
Use (T-scores) by Stage
55
Pros
50
Cons
45
40
PC n=113
C n=257
P n=124
N=828
A n=143
M n=191
55
50
45
60
Baseline Sample - Confidence in Condom
Use (T-scores) by Stage
40
PC n=113
C P n=257 n=124
N=828
A n=143
M n=191
TTM-Tailored Expert Systems for Condom Use & Smoking
For use in Family Planning Clinics
Mouse input (no keyboard!)
On-screen and printed feedback
Printed feedback for both participant and her clinic counselor
Smoking system appropriate for both smokers (cessation) and nonsmokers
(prevention)
TTM Tailored Intervention
Package
Interactive assessment and expert system feedback (onscreen & printed)
Condom Use Promotion
Smoking Cessation OR Prevention
Tailored feedback based on:
Stages of change
Pros & Cons
Confidence or Temptation
Processes of Change
Stage-Matched Counseling
Standard Care Intervention
Package
Identical computer-delivered assessment and generic feedback to use condoms, condom tips, and advise to either quit smoking or avoid starting to smoke.
Standard family planning counseling on birth control and condom use.
Stage-matched Counseling
Can be used with teens at all stages of change, not only those ready for action
Comparable to Motivational Interviewing
Counselors match Process exercises to stage using Manual
Counselor received printed output from computer with client’s stage of change and processes to work on
Processes of Change
• HOW people change
• cognitive, emotional, behavioral, interpersonal strategies/techniques used to change behavior
• different processes mediate transitions between stages
• process-to-outcome research
• foundation of intervention design
Processes of Change
Experiential
Processes
Behavioral
Processes
Thinking, Feeling or
Experiencing
Consciousness Raising
Dramatic Relief
Environmental Reevaluation
Self Reevaluation
Social Liberation
Doing
Counterconditioning
Helping Relationships
Reinforcement Management
Self Liberation
Stimulus Control
Newer Interpersonal
Processes
Condom Communication - talking about condom use
Condom Assertiveness - insisting on condom use
Eroticizing Condoms - finding ways of making using condoms more enjoyable
Partner Support - getting partner’s support for condom use
Interpersonal Systems Control - avoidance of challenging people and/or social/sexual situations
Experiential Processes of Change
For Condom Use By Stage
40
35
50
45
60
55
CR
DR
ER
SO
SR
P
N=113
C
N=257
D
N=124
A
N=143
M
N=191 N=828
Interpersonal Processes of Change By Stage
60
55
50
45
40
35
P
N=113
C D
N=257
N=124
N=828
A
N=143
M
N=191
AS
CO
EC
PS
Retention Rates
Assessment/ Intervention
Baseline
Time 2
Time 3
Time 4
12 months
18 months
N
833
470
437
442
530
500
%
100
56.4
52.5
53.1
63.6
60.0
% A/M - Condom Use in Baseline nonusers by Group by Time
50
25
20
15
10
5
0
45
40
35
30
Baseline 6 months 12 months 18 months
TTM
Std. Care
% A/M – ITT Condom Use by Group by Time
(N=494)
30
25
20
15
10
5
0
Baseline 6 months 12 months 18 months
TTM
Std. Care
% A/M - Baseline condom users by group by time
120
100
80
60
40
20
0
Baseline 6 months 12 months 18 months
TTM
Std. Care
Quit Rates in Smokers by Group at 18 months
(n=88, ns)
40
30
20
10
0
18 Months
TTM
SC
Smoking Uptake among Baseline
Nonsmokers by Group
20
15
10
5
0
TTM
Std. Care
18 months
Step by Step Conclusions
Results support the efficacy of the TTM Tailored expert system intervention & stage matched counseling package to increase condom use and reduce condom relapse in this high risk sample
Despite lack of statistical significance, smoking cessation results at 18 months replicated prior results with adults and adolescents.
No support for effectiveness of the smoking prevention intervention.
Significant initial increases in condom use were sustained over 18 months, however, control group caught up.
Remaining Questions?
Would these results generalize to at risk adults?
Would condom use results hold up without the counseling component?
Tailored intervention to increase dualmethod use: an RCT to reduce unintended pregnancies and STIs
Peipert JF 1 , Redding CA 3 , Blume JD 2 ,
Allsworth JE 1 , Matteson KA 2,4 , Lozowski
F 2,4 , Mayer KH 2 , Morokoff PJ 3 , Rossi JS 3
1- Washington University, School of Medicine, St. Louis, MO
2 - Brown University, Providence, RI
3 - University of Rhode Island, Kingston, RI
4 - Women and Infants Hospital, Providence, RI
5 - Rhode Island Hospital, Providence, RI
Project PROTECT Study
Dual Method Use
Recruited N=542 at risk women (13-35)
59% of eligibles recruited
Tested for STIs before enrollment
If +, treatment & test of cure before enrollment
English speaking
Avoid pregnancy X 2 years
< 13 y.o. required parental consent
RCT
PROTECT Study Timepoints
Baseline – full exam
TTM group - 1 + 2 months sessions
Standard Care – no additional sessions
6 & 18 months phone survey
12 & 24 months – full survey & exam
PROTECT Baseline Sample
Characteristics (N=542)
Median Age = 22 years
90% Single
25% < H.S. Education (*unbalanced)
22% Black & 17% Hispanic
47% History STI (*unbalanced)
49% History unplanned pregnancy
34% No contraceptive use
33% Hormonal contraceptive use
48% smokers
PROTECT Study Outcomes
TTM
N=272 n (%)
Reported
Dual Method
Use
86 (32)
Reported
Consistent
Condom Use
124 (46)
Any STI or unintended pregnancy
95 (35)
Control
N=270 n (%)
71 (26)
124 (46)
93 (34)
Unadjusted
HRR
Adjusted for propensity score
(95% CI)
1.38
(1.00, 1.89)
1.70
(1.09, 2.66)
1.14
(0.89, 1.47)
1.26
(0.88, 1.79)
1.08
(0.81, 1.44)
1.19
(0.79, 1.79)
PROTECT Conclusions
TTM Tailored Expert system increased reported dual method use (~ 70%)
Smaller effect on condom use (~ 30% increase) – not significant
No effect on incident STIs and unplanned pregnancies
RI Project RESPECT
CA Redding 1 , PJ Morokoff 1 , JS Rossi 1 , KS Meier 1 ,
BB Hoppner 1 , K Mayer 2 , B Koblin 3 , P Brown-
Peterside 3
1 – Psychology Department & CPRC, University of RI
2 - Miriam Lifespan Hosp. & Brown Univ., Providence, RI
3 – New York Blood Center, Bronx, NY
RI Project RESPECT
9 local sites in urban areas
Drug Tx. Programs, STD Clinics
1 site in the Bronx, NY Blood Center
RCT
TTM-Tailored ES Feedback compared to
Generic feedback alone
Intervention at Baseline, 2, 4 months
Follow-up at 6, 12, 18 months
Participation Criteria
18 - 44 years old & English speaking
Heterosexually active in past 3 months
unprotected vaginal or anal sex
At least one opposite sex partner
Not pregnant or trying to get pregnant
Self report - HIV Negative
Participation Criteria continued
One of the following in the past year:
3 sexual partners
diagnosed with an STI (other than HIV)
a sex partner with 3 sex partners
a sex partner who is a bisexual male
a sex partner who has injected drugs
exchanged sex for money or drugs
Baseline Sample (n=315)
Age mean = 32.2 years (s.d. = 8.1)
Gender
28% Male
Employment
65% Unemployed
21% Full-time work
11% Part-time work
3% Other
Education
42.5% < H.S.
More Sample Description
Diversity (matches rates of HIV in RI)
38% White
33% African American
23% Hispanic
Relationship Status
86% Unmarried/Separated/Divorced/Widowed
14% Married or Living With Partner
85% Sexually Active in past 2 months
Behavioral Risks
Hx. Of STI
Used injection drugs
Not use condom @ last sex
Age of sexual debut
# sex partners in past 30 days
%
46.4
18
72
15.25
3.2
Baseline Stages of Condom Adoption
(N=315) At-Risk Sample of M + F
45
40
35
30
25
20
15
10
5
0
PC
40%
C
41%
PR
14%
A
5% -
M
Expert System Enhancements
New background pictures + recorded new adult male and female audio.
Gender-matched systems
Added new sections for main and other partner readiness to use condoms.
Respect Retention Rates
Assessment/ Intervention
Baseline
2 months
4 months
6 months
12 months
18 months
N %
527 100
409 77.6
338 64.1
359 68.1
324 62.2
278 52.8
Outcomes
-
-
- DVs:
# times unprotected sex in past 30 days
(n = 267)
% of times safe (includes those not sexually active in past 30 days)
(n=296)
% A/M consistent condom use
(N=292)
Any Stage Progress
(N=305)
9
7
5
3
1
15
13
11
Baseline to 6 Months X Group -
# times unprotected sex (N=267)
Treatment
Control
Baseline 6-Month
6 Mos. - % Time Being Safe
N=296
100%
80%
60%
40%
20%
0% treatment control
Baseline 6-Month
% A/M at 6 months
(n=292)
30%
25%
20%
15%
10%
5%
0%
0.246
0.14
Treatment Control
Includes only Pre-Action Stages at Baseline (PC, C, PR)
10% more progress to A/M in Tx than in Control
Intent to Treat (ITT) Analysis of
% A/M at 6 months
(n=448)
30%
25%
20%
15%
10%
5%
0%
0.162
0.091
Treatment Control
All baseline pre-action S’s included.
Assumes no progress among lost-to-follow-up participants.
Reduces effect from 10% to 7%, still statistically significant.
Any Stage Progress at 6 months
(n=305)
60%
50%
40%
30%
20%
10%
0%
0.551
0.451
Treatment Control
10% more progress in Treatment than in Control
ITT Analysis of Stage Progress
(N=448)
60%
50%
40%
30%
20%
10%
0%
0.357
0.287
Treatment Control
~ 7% more progress in Tx than in Control
RI Project RESPECT
Conclusions
We were able to recruit a high risk sample of men and women from different sites.
We were able to get good proportions (78%) of the sample to come back for at least 2 sessions.
Retention was a concern.
Results support the 6 month efficacy of the TTM
Tailored expert system to increase condom use in this high risk sample.
Longer term outcomes look like Step X Step…
Durability of these effects over time?
Differences Across Studies?
Condom use STI
Study N d [95% CI] d [95% CI]
Protect 346 0.140 [0.07
–0.35] 0.154 [0.06
–0.36]
RI Respect 292 0.461 [0.23
–0.69]
Step By Step 622 0.477 [0.32
–0.64]
Noar SM, Black HG, Pierce LB. (2009). Efficacy of computer technology-based
HIV prevention interventions: a meta-analysis. AIDS , 23 , 107–15.
What’s next?
Process to outcome research
Compare cross-sectional to longitudinal findings
Examine predictors of changes over time
Enhance intervention outcomes
(More sessions? New variables? New behaviors?
More ? )
Enhance retention
Generalize to additional at risk samples + settings
Dissemination & Translation
Useful Intervention Refinement
Process
Focus Groups/ Formative Work
New questions?
Pilot Sample - Measurement work
Efficacy/effectiveness trials
Pilot test intervention